-
Osteoporosis (OP) is a metabolic bone disease characterized by imbalanced bone formation and resorption rates, leading to bone loss and predisposition to fracture[1]. The incidence of OP is gradually increasing as the population ages, seriously affecting older adults and postmenopausal women[2]. The proliferation and differentiation of human bone-marrow-derived mesenchymal stem cells (hBMSCs) are closely related to bone metabolism[3]. hBMSCs are mainly located in the bone marrow cavity and are multipotent stem cells with the potential for self-renewal and multidirectional differentiation (into adipocytes, chondrocytes, myoblasts, and osteoblasts)[4,5]. Therefore, promoting the osteogenic differentiation (OD) of hBMSCs is of great clinical importance in the treatment of OP[6].
The root of the traditional Chinese herb Astragalus (AST) has been used medicinally for more than 2,000 years for its anti-inflammatory, anticancer, and antidiabetic properties[7]. Increasing evidence suggests that AST and its main active constituents play therapeutic roles in OP. For example, AST inhibits OP in mice[8]. AST and raspberry can be used for the clinical prevention and cure of postmenopausal OP[9]. Although a partial role of AST in OP has been demonstrated, its specific intrinsic mechanism has not been fully elucidated.
MicroRNAs are single-stranded noncoding RNAs controlling post-transcriptional gene expression by combining with the 3′ untranslated region (UTR) of target gene mRNAs[10]. miRNAs are involved in the progression of OP, particularly in the differentiation and function of osteoblasts and osteoclasts[11]. For example, miR-187 stimulates bone remodeling and healing in a mouse model of OP to promote the OD of human pluripotent stromal cells[12]. The miRNA let-7a-5p participates in osteoblast differentiation to mitigate the development of OP[13]. Previous studies have found that the main active ingredient of AST, astragaloside IV, is multi-targeted and may be effective in the treatment of diseases by altering miRNAs to regulate different pathways[14-16]. Astragaloside IV has been shown to ameliorate tibial defects and promote the proliferation and osteogenic differentiation of hBMSCs in rats by modulating the miR-124-3p.1/signal transducer and activator of transcription 3 axis[17]. In addition, astragaloside IV promotes the osteogenic differentiation of BMSCs through the miR-21/neuron growth factor/bone morphogenetic protein-2/runt-related transcription factor 2 (Runx2) pathway[18]. Therefore, we hypothesized that AST mediates the progression of OP by regulating miRNAs. miR-181d-5p, a newly identified miRNA, is upregulated during fracture healing[19]. Downregulation of miR-181d-5p promotes the OD of BMSCs by increasing the expression level of the osteogenic transcription factor Runx2[20]. However, its expression and function in OP remain unclear.
This study aimed to determine the function of AST in OP and its effect on the OD of hBMSCs. We hypothesized that AST may mitigate OP by controlling the OD of hBMSCs through the regulation of miR-181d-5p, possibly offering a new basis for OP treatment.
-
Astragaloside IV was used as a marker for the quality control of AST and its products in the Chinese Pharmacopoeia (2015 edition)[21]. In this study, the quality of the AST products used met the Chinese Pharmacopoeia (2015 edition) standards and were obtained from the Tianjiang Yifang Company (Kunming, Yunnan, China). The astragaloside IV content was determined using high-performance liquid chromatography (HPLC), and it was found that the lyophilized powder of the AST extract used in this study contained 0.20% astragaloside IV. The HPLC conditions were as follows: column, Utrasphere-ods 5 μm 4.6 mm × 250 mm; mobile phase, acetonitrile:0.1 phosphoric acid; flow rate, 1 mL/min, detection wavelength, 203 nm.
Dried AST was weighed, mixed with 10 times distilled water (volume), boiled for 1 h, preserved in water for 30 min, and filtered through gauze. The residue was diluted eight-fold volume in distilled water and boiled for 1 h. After filtration, the two decoctions were mixed and concentrated via centrifugation in a rotary evaporator. The crude decoction had an AST concentration of 1.0 g/mL.
-
Primary hBMSCs were isolated from patients undergoing hip replacement, as described previously[22]. The hBMSCs were isolated from the bone marrow, separated by density gradient centrifugation in Ficoll-Histopaque (d = 1.077 g/mL; Pharmacia, Uppsala, Sweden), and cultured in proliferation medium (PM) composed of Dulbecco’s modified Eagle’s medium (Gibco, Waltham, MA, USA), 10% (v/v) fetal bovine serum (ScienCell, Carlsbad, CA, USA) and 1% (v/v) penicillin/streptomycin (Gibco). To induce OD, hBMSCs in passage 3 were seeded in 12-well plates at 5 × 105/well and grown to 80% confluence. Osteogenic medium (OM) containing PM, 10-mmol/L β-glycerophosphate, 100-nmol/L dexamethasone, and 0.2-mmol/L L-ascorbic acid (Sigma-Aldrich, St Lois, MO, USA)[23] was added. AST (80, 160, or 320 mg/mL) was then added to the medium and the cells were incubated for 48 h.
-
Oligonucleotides (negative controls, miR-181d-5p inhibitor [339121]/mimic [339173], and SOX11-overexpression plasmid) were obtained from Qiagen (Duesseldorf, Germany). A short hairpin RNA specific for SOX11 (sh-SOX11) (sense sequence: GCCTCTACTACAGCTTCAAGA; antisense sequence: TCTTGAAGCTGTAGTAGAGGC) and a negative control (sh-NC) were purchased from GenePharma (Shanghai, China). Transfection was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA)[24].
-
Transfected or untransfected hBMSCs (2 × 104/mL, 150 μL) were seeded in 96-well plates and cultured in OM with or without AST for 72 h. MTT solution (5 mg/mL, 20 μL; M1020; Solarbio, Beijing, China) was added and the cells were incubated for 4 h, followed by supplementation with 150 μL of dimethyl sulfoxide for 10 min incubation. The absorbance was measured at 490 nm using a Multiskan Spectrum spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
-
After 7 d of OD, hBMSCs were fixed in 4% paraformaldehyde for 30 min, stained with 0.1% ARS solution (HZ121105-100; Shanghai Huzhen Industrial Co., Ltd, Shanghai, China) for 0.5 h – 2 h, and observed under an inverted phase-contrast microscope. The area of mineralized nodules was calculated using Image Pro Plus 6.0 (Media Cybernetics, Silver Springs, MD, USA)[25].
-
After 7 days of OD, ALP activity was determined using the ALP Diethanolamine Activity Kit (Yeasen Biotech, Shanghai, China). After 24 h of staining, the hBMSCs were collected and lysed using cold lysis buffer. The collected supernatant was centrifuged at 2,500 × g for 15 min and mixed with p-nitrophenyl phosphate SensoLyte. The optical density was measured at 570 nm using a microplate reader (Bio-Tek Instruments, Winooski, VT, USA)[26].
-
To analyze apoptosis, cells were double-stained using a PE Annexin V Apoptosis Detection Kit (BD Pharmingen, Franklin Lakes, NJ, USA). Stained cells were immediately loaded onto a flow cytometer (BD Pharmingen).
To determine the cell cycle stage, the cells were fixed in 75% ethanol overnight at 4 °C, after which 500 μL of propidium iodide (BD Pharmingen) was added and the cells were incubated at 4 °C for 30 min protected from light. Finally, the cells were detected using a flow cytometer (BD Pharmingen)[27].
-
Female Sprague-Dawley rats, weighing 280 g – 300 g, were obtained from Shanghai Slack Laboratory Animal Co., Ltd. (Shangahi, China). An OP rat model was established using ovariectomy (OVX), and laparotomy was performed under pentobarbital sodium (30 mg/kg) anesthesia. Both ovaries were removed and sutured layer by layer. The rats were administered an intramuscular injection of 20,000 U of penicillin for 3 days. Incompletely ovariectomized rats identified using vaginal smear examination were removed 5 days after OVX. The abdominal cavity of sham rats was incised and sutured. Each group received the corresponding drug intervention, whereas the control group received the same amount of distilled water daily for 12 weeks. One week later, the rats were euthanized after intraperitoneal anesthesia with ketamine and hydroxyzine, and femur tissues were collected[28].
The rats were divided into the following nine groups, with eight rats in each group: sham group, OVX group, low-dose AST group (2.4 g/kg), medium-dose AST group (4.8 g/kg), high-dose AST group (9.6 g/kg), high-dose AST (9.6 g/kg) + agomir NC group (9.6 g/kg AST + tail vein injection of agomir NC [10 mg/kg per week, 12 weeks]), high-dose AST + miR-181d-5p agomir group (9.6 g/kg AST + tail vein injection of agomir-miR-10a-3p [10 mg/kg per week, 12 weeks]), high-dose AST + sh-NC group (9.6 g/kg AST + tail vein injection of sh-NC [10 mg/kg per week, 12 weeks]), and high-dose AST + sh-SOX11 group (9.6 g/kg AST + tail vein injection of sh-SOX11 [10 mg/kg per week, 12 weeks]). Decoctions prepared at high, medium and low doses were administered by gavage at 10 μL/g. The intervention lasted for 12 weeks and the rats were weighed weekly. The dose was adjusted according to changes in body weight.
-
Rat femurs were fixed in 4% paraformaldehyde for 24 h and scanned using a micro-CT scanner (Siemens, Munich, Germany) at 80 kV and 500 mA. The scanning time and resolution were 800 ms/frame and 10.44 μm, respectively, for 360-degree scanning in 0.5 increments. The region of interest (2.5 × 2.5 × 3 mm3) was located 1,500 μm above the proximal epiphyseal growth plate. Bone mineral density (BMD), bone volume-to-total volume ratio (BV/TV), trabecular bone number (TbN), and trabecular bone thickness (Tb. Th) were recorded. Micro-CT analysis was performed using COBRA software[29].
-
Rat femurs were fixed with 4% paraformaldehyde for 24 h, decalcified, and embedded in paraffin. Sections were cut at a thickness of 4 μm and HE staining was performed[30].
-
After deparaffinization, the sections were stained with hematoxylin and Masson’s trichrome stain, treated with 2% glacial acetic acid, and hydrated with 10% molybdic acid. After staining with aniline blue, the sections were washed with an alcohol gradient, mounted with neutral gum, and viewed under an optical microscope[31].
-
Tissue sections (thickness 4 μm) were dewaxed and treated with xylene and ethanol. After antigen retrieval, the sections were blocked with goat serum, followed by incubation with a primary antibody against osteocalcin (OCN; ab93876; 5 µg/mL; Abcam, Cambridge, UK) and a horseradish peroxidase (HRP)-conjugated secondary antibody. A diaminobenzidine detection kit (ZSGB-BIO, Beijing, China) was used to detect the immunoreactive signals. Finally, tissue sections were observed under a light microscope.
-
Total RNA was extracted from cells using TRIzol reagent (Invitrogen). After checking the purity and concentration of the RNA samples using a UV spectrophotometer, reverse transcription was performed using a RevertAid First Strand cDNA Synthesis kit (Thermo Scientific). PCR was performed using SYBR Green qPCR Master Mix (Thermo Fisher Scientific). The primer sequences are listed in Table 1[32].
Table 1. Primer sequence
Genes Primer sequence (5'-3') miR-181d-5p Human F: 5'-GGCAGAACATTCATTGTTGTCG-3' R: 5'-GCAGGGTCCGAGGTATTC-3' Rat F: 5'-GGCAGAACATTCATTGTTGTCG-3' R: 5'-GCAGGGTCCGAGGTATTC-3' U6 Human F: 5'-CTCGCTTCGGCAGCACA-3' R: 5'-AACGCTTCACGAATTTGCGT-3' Rat F: 5'-CTCGCTTCGGCAGCACA-3' R: 5'-AACGCTTCACGAATTTGCGT-3' SOX11 Human F: 5'-CCAGGACAGAACCACCTGAT-3' R: 5'-CCCCACAAACCACTCAGACT-3' Rat F: 5'-CAGCGAGAAGATCCCGTTCA-3' R: 5'-GTCCGTCTTGGGCTTTTTGC-3' RUNX2 Human F: 5'-GCGCATTCCTCATCCCAGTA-3' R: 5'-GGCTCAGGTAGGAGGGGTAA-3' Osterix Human F: 5'-GATGGCGTCCTCTCTGCTTG-3' R: 5'-AATGGGCTTCTTCCTCAGCC-3' GAPDH Human F: 5'-CACCCACTCCTCCACCTTTG-3' R: 5'-CCACCACCCTGTTGCTGTAG-3' Rat F: 5'-GTCGGTGTGAACGGATTTG-3' R: 5'-TCCCATTCTCAGCCTTGAC-3' Note. F, forward; R, reverse; U6, U6 small nuclear RNA; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. -
Total protein was extracted using radioimmunoprecipitation assay lysis buffer (Beyotime Biotechnology, Shanghai, China) and quantified using a bicinchoninic acid kit (Beyotime Biotechnology). The target proteins were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA), and incubated with 5% non-fat milk diluted in 0.1% Tris-buffered saline. The membranes were then incubated with rabbit primary antibodies against human SRY-box transcription factor 11 (SOX11) (1:1,000; HPA000536; Millipore Sigma), RUNX2 (1:1,000; ab23981; Abcam), Osterix (1:1,000; ab22552; Abcam), and glyceraldehyde-3-phosphate dehydrogenase (1:2,500; ab9485; Abcam) (all from Abcam). HRP-conjugated goat anti-rabbit immunoglobulin G (IgG) (12,000; ab205718; Abcam) was then added, and protein bands were detected using enhanced chemiluminescence (Bio-Rad)[33].
-
Bilateral femurs and tibias were harvested from Sprague-Dawley rats under aseptic conditions. The medullary cavity was flushed with low-glucose Dulbecco’s modified DMEM medium (Gibco). The bone marrow tissue was centrifuged at 1,000 rpm for 5 min to remove suspended adipose tissue. Bone marrow precipitates were then resuspended in complete L-DMEM containing 10% FBS and 1% penicillin and streptomycin and cultured at 37 °C/5% CO2. After 48 h, the non-adherent cells were removed by replacing the medium. The medium was replaced every 3 days. When the cells reached 80%–90% confluence, they were trypsinized, counted, and reseeded as the first passage. Cells from passages to 3–6 were used for subsequent experiments.
-
PmirGLO-SOX11 wild-type (SOX11-wt) and pmirGLO-SOX11 mutant (SOX11-mut) plasmids were produced by cloning wild-type and mutant SOX11 fragments containing the predicted miR-181d-5p binding sites in the pmirGLO luciferase reporter assay vector (Promega, Madison, WI, USA), respectively. hBMSCs or rBMSCs (in 48-well plates, 3 × 105/well) were co-transfected with the plasmid and miR-181d-5p/NC using Lipofectamine 3000. Luciferase activity was measured using the Dual-Luciferase Reporter Gene Detection Kit (Promega).
-
RIP experiments were performed using the RIP™ Kit (Millipore). Immunoprecipitation was performed using antibodies against normal mouse immunoglobulin G (IgG) and human Ago2. hBMSC or rBMSC lysates were incubated with antibody-conjugated magnetic beads in RIP buffer and analyzed using real-time PCR[34].
-
Data were analyzed using SPSS software (version 21.0; IBM, Armonk, NY, USA). According to the Kolmogorov-Smirnov test, the data were normally distributed and are expressed as the mean ± standard deviation. Student’s t-tests were used for comparisons between two groups, and analysis of variance and Fisher’s least significant difference t-tests were used for comparisons between multiple groups. Enumeration data are expressed as rates or percentages and compared using the chi-square test. Two-sided P values < 0.05 were considered statistically significant.
-
The OD of BMSCs was induced and the cells were treated with different doses of AST. The viability of hBMSCs was enhanced under osteogenic conditions and AST further increased the viability of hBMSCs in a concentration-dependent manner (Figure 1A). Analyses of the cell cycle distribution and apoptosis indicated that the number of G0/G1 phase cells was reduced after the OD of hBMSCs, and AST further reduced the number of G0/G1 phase cells (Figure 1B). AST also reduced the apoptotic rate of hBMSCs (Figure 1C). The mineralization ability of hBMSCs was elevated after OD and further improved after the dose-dependent addition of AST (Figure 1D). ALP activity in hBMSCs was elevated under osteogenic conditions, and AST further enhanced the ALP activity (Figure 1E). RUNX2 and Osterix are well-known OD markers. RUNX2 and Osterix mRNA and protein expression levels were enhanced after OD, and AST increased RUNX2 and Osterix expression (Figure 1F, G). Notably, during the OD of hBMSCs, miR-181d-5p downregulation was detected, whereas after the addition of AST, miR-181d-5p expression levels decreased with increasing doses (Figure 1H). A high dose was chosen for follow-up experiments.
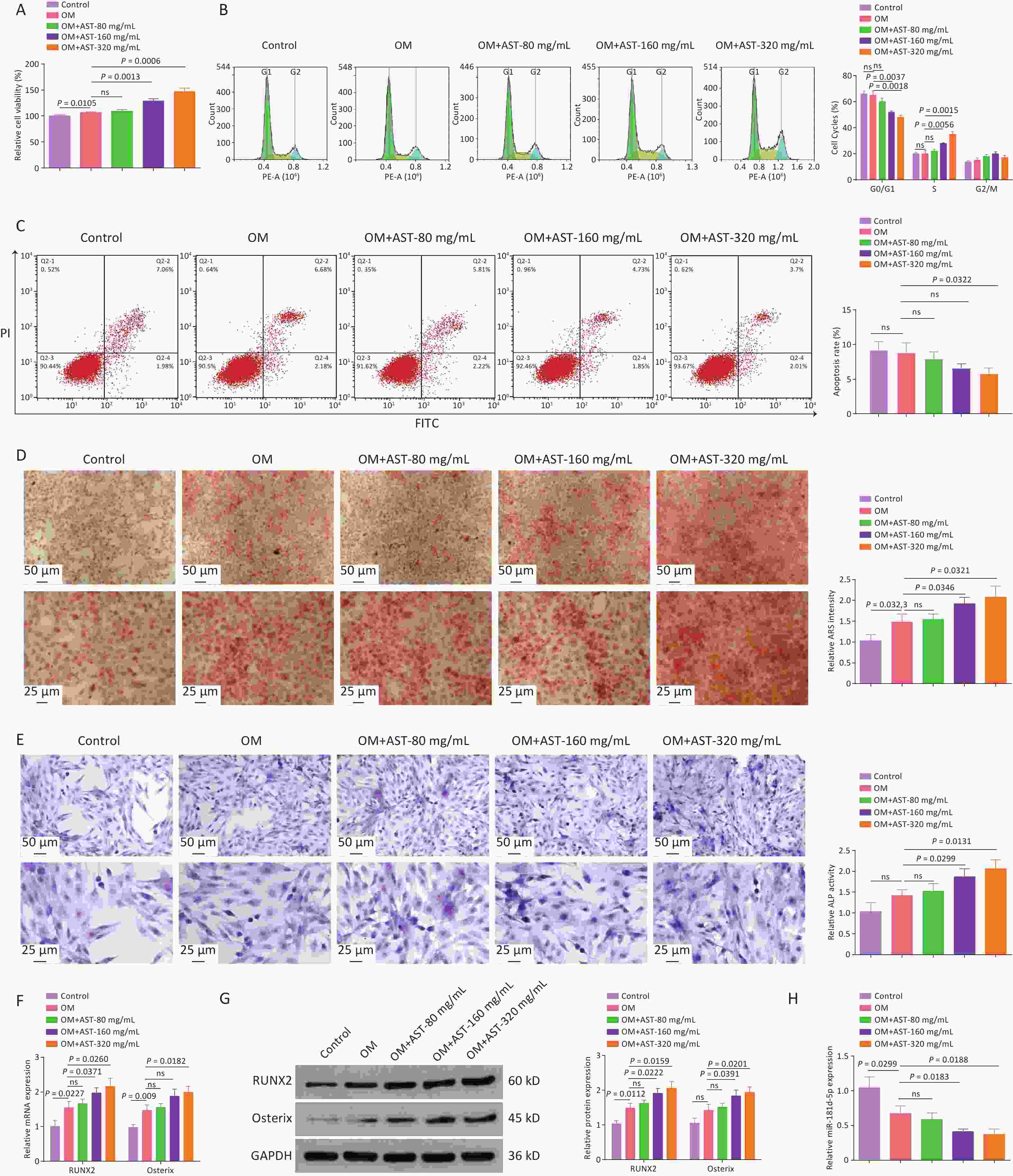
Figure 1. AST promotes the OD of BMSCs and reduces miR-181d-5p levels in vitro. (A) 3-MTT assay for the detection of hBMSC viability; (B–C) Flow cytometry test of the cell cycle and apoptosis; (D) ARS staining of hBMSCs; (E) ALP staining test of ALP activity; (F–G) qRT-PCR and western blotting assays of RUNX2 and Osterix expression; (H) qRT-PCR analysis of miR-181d-5p levels. N = 3. OD, osteogenic differentiation; MTT, 4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; OM, osteogenic medium; hBMSC, human bone marrow-derived mesenchymal stem cell; AST, astragalus; ARS, Alizarin Red S; ALP, alkaline phosphatase; qRT-PCR, quantitative reverse transcription polymerase chain reaction; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
-
After AST treatment, miR-181d-5p mimics and inhibitors elevated and reduced miR-181d-5p expression levels, respectively (Figure 2A). Subsequent experiments revealed that the elevation of miR-181d-5p levels impaired the stimulatory effect of AST on the OD of hBMSCs, reduced cell viability, blocked cells in the G0/G1 phase, and increased apoptosis, whereas inhibiting miR-181d-5p had the opposite effects (Figure 2B–D). Meanwhile, elevating miR-181d-5p levels weakened the cell mineralization capacity and reduced ALP activity and RUNX2 and Osterix expression levels, whereas inhibiting miR-181d-5p had the opposite effect (Figure 2E–H).
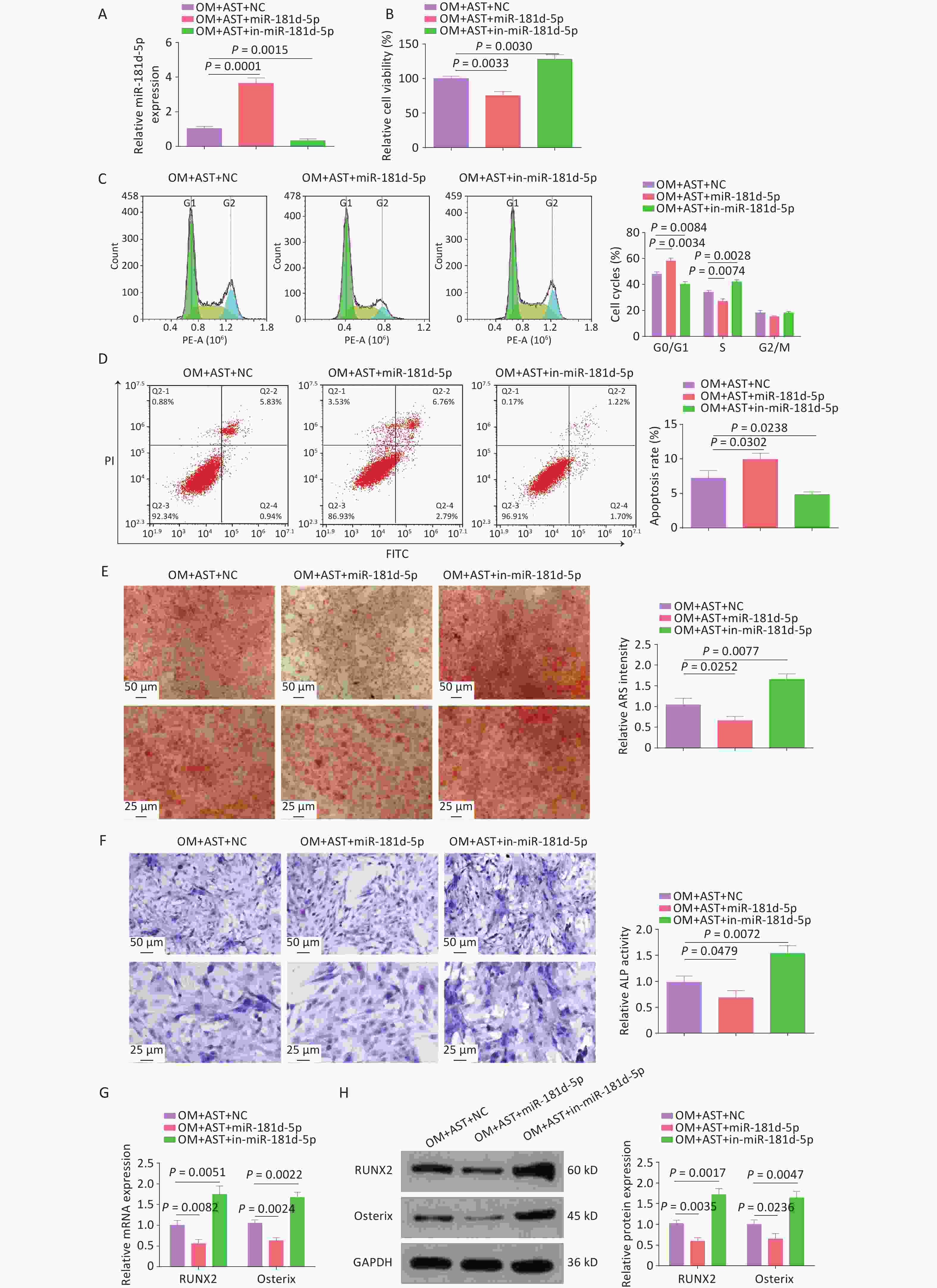
Figure 2. Increased miR-181d-5p levels reduce the promoting effect of AST on the OD of hBMSCs. (A) qRT-PCR analysis of miR-181d-5p after regulating miR-181d-5p levels; (B) MTT assay for the detection of hBMSC viability after regulating miR-181d-5p levels; (C–D) Flow cytometry detection of the cell cycle stage and apoptosis after regulating miR-181d-5p levels; (E) ARS of hBMSCs after regulating miR-181d-5p levels; (F) ALP staining test of ALP activity; (G–H) qRT-PCR and western blotting assays of RUNX2 and Osterix after regulating miR-181d-5p levels. N = 3. OM, osteogenic medium; AST, astragalus; NC, negative control; in, inhibitor; ARS, Alizarin Red S; ALP, alkaline phosphatase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
-
Of note, elevated SOX11 expression levels were also detected during the OD of hBMSCs and they were increased after AST treatment (Figure 3A, B). The overexpression or inhibition of miR-181d-5p resulted in decreased or increased expression levels of SOX11, respectively, in hBMSCs (Figure 3C, D). The binding sites of miR-181d-5p and SOX11 were then tested (Figure 3E). The overexpression of miR-181d-5p reduced the luciferase activity of wild-type SOX11, but had no distinct effect on that of mutant SOX11 (Figure 3F). Moreover, the RIP assay further confirmed the binding of miR-181d-5p to SOX11 in anti-Ago2 precipitates (Figure 3G).
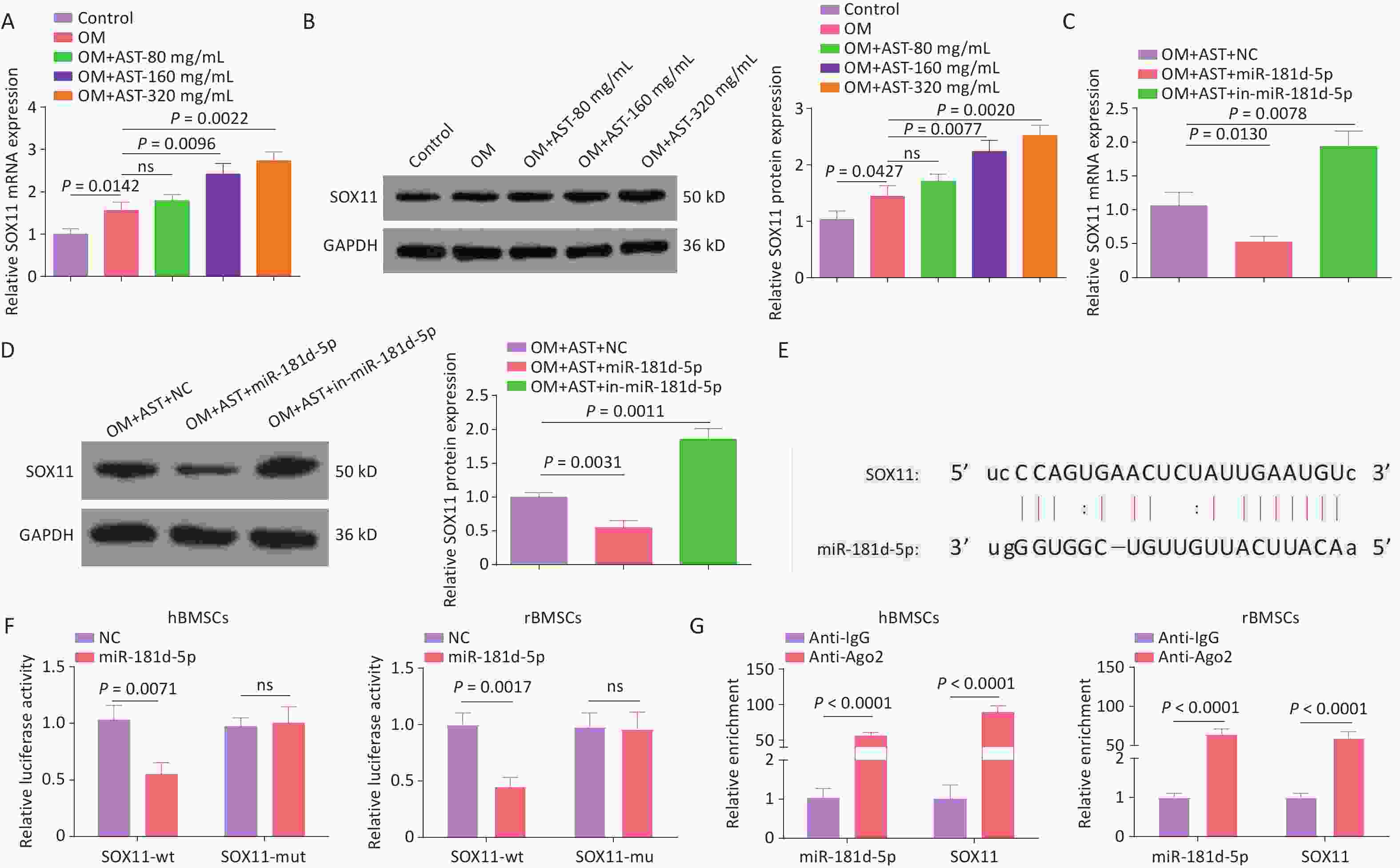
Figure 3. miR-181d-5p Targets SOX11 In hBMSCs. (A–D) qRT-PCR and western blotting detection of SOX11 in hBMSCs; (E) Bioinformatics website prediction of the binding site of miR-181d-5p and SOX11; (F) Luciferase activity assay to verify the relationship between miR-181d-5p and SOX11; (G) RIP assay to confirm the binding of miR-181d-5p to SOX11. N = 3. OM, osteogenic medium; AST, astragalus; NC, negative control; in, inhibitor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; wt, wild-type; mut, mutant; ns, not significant; RIP, RNA immunoprecipitation.
-
AST-treated hBMSCs were transfected with plasmids overexpression or knocking down (oe-SOX11, sh-SOX11), and their respective negative controls, to alter SOX11 expression (Figure 4A). After SOX11 overexpression, the effect of AST on the OD of hBMSCs was enhanced; that is, the activity of hBMSCs was further increased; the number of cells in the G0/G1 phase was reduced; and apoptosis, the number of calcium nodules, ALP activity, and RUNX2 and Osterix expression levels were decreased. After SOX11 inhibition, the effect of AST on the OD of hBMSCs was offset (Figure 4B–H).
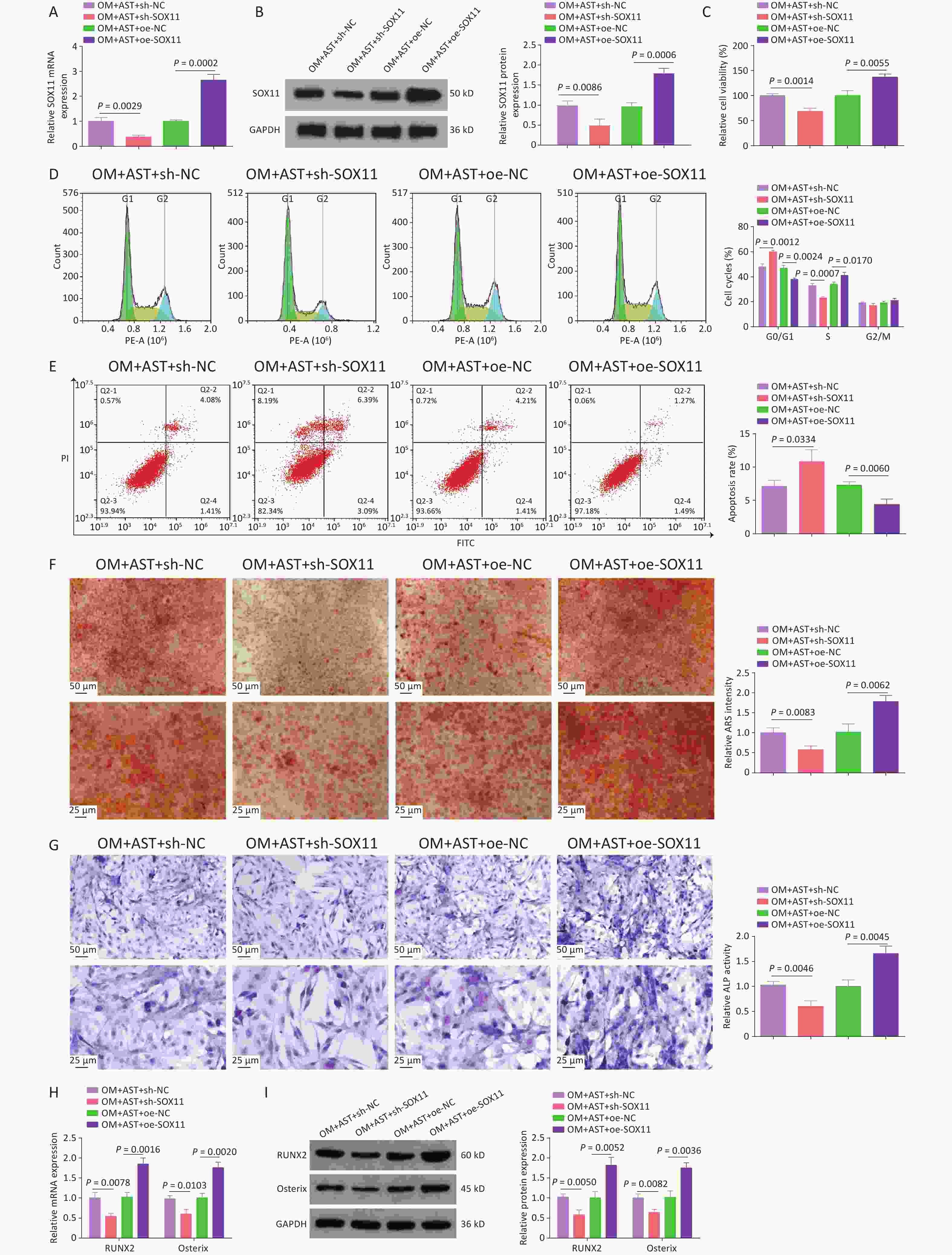
Figure 4. SOX11 Further Promotes the Effect of AST on the OD of hBMSCs. (A–B) qRT-PCR and western blotting detection of SOX11 after regulating SOX11; (C) MTT detection of hBMSC viability after regulating SOX11; (D–E) Flow cytometry detection of the cell cycle stage and apoptosis after regulating SOX11; (F) ARS of hBMSCs after regulating SOX11; (G) ALP staining test of ALP activity; (H–I) qRT-PCR and Western blotting assays of RUNX2 and Osterix after SOX11 regulation. N = 3. OM, osteogenic medium; AST, astragalus; sh, short hairpin RNA; NC, negative control; oe, overexpression; ARS, Alizarin Red S; ALP, alkaline phosphatase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
-
To further investigate the molecular mechanism of miR-181d-5p/SOX11 in the OD of hBMSCs, miR-181d-5p and SOX11 were overexpressed in AST-treated hBMSCs (Figure 5A). After the overexpression of miR-181d-5p and SOX11, the OD process of hBMSCs returned to the level seen with AST treatment alone. Overexpression of SOX11 weakened the effect of miR-181d-5p overexpression on cell viability, apoptosis, the number of G0/G1 phase cells, mineralization capacity, ALP activity, and RUNX2 and Osterix expression levels (Figure 5B–H).
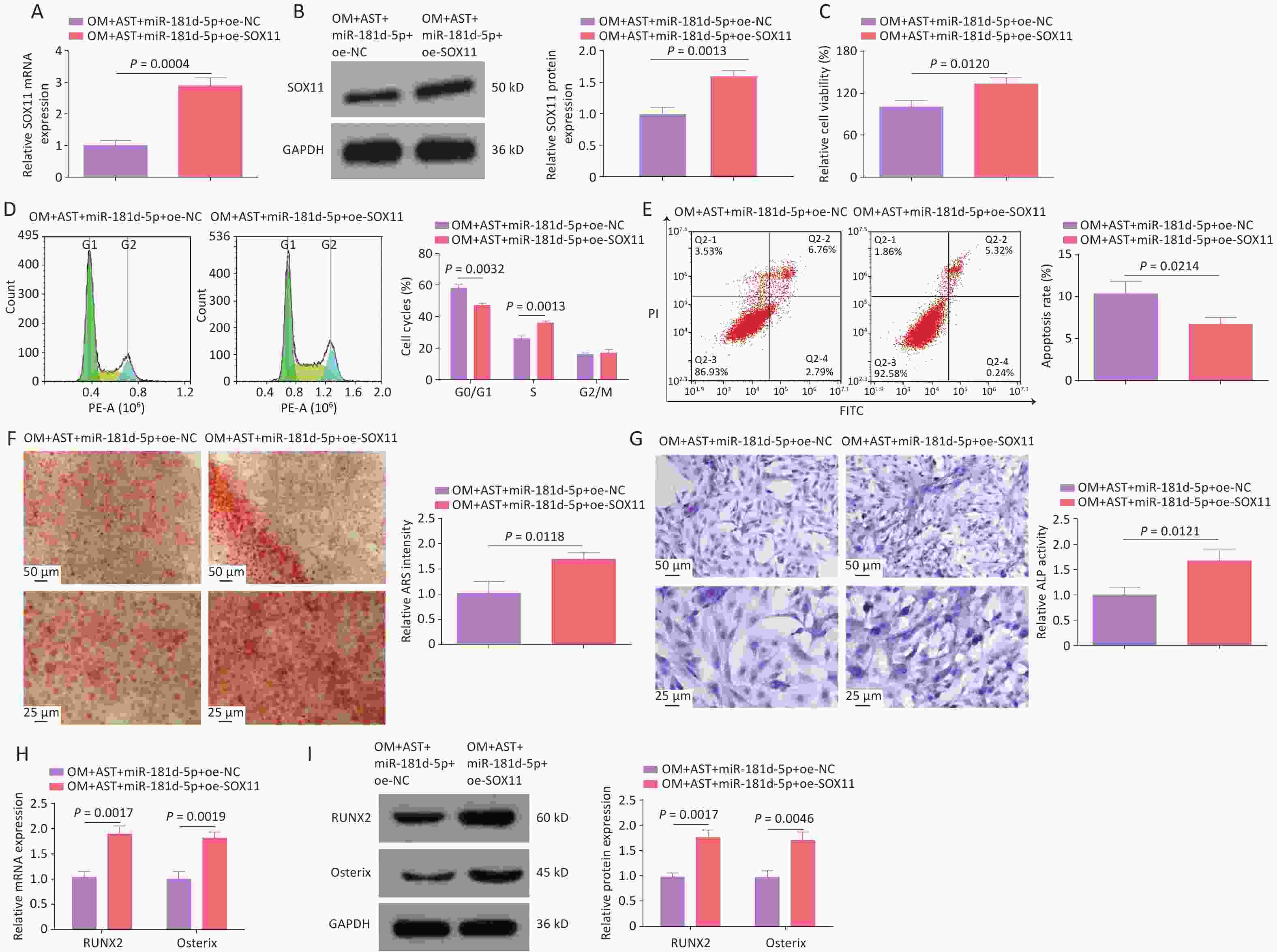
Figure 5. SOX11 reversed the inhibition of hBMSCs OD mediated by miR-181d-5p. (A–B) qRT-PCR and western blotting detection of SOX11 after regulating SOX11; (C) MTT detection of hBMSCs cell viability after regulating SOX11; (D–E) Flow cytometry detection of the cell cycle stage and apoptosis of hBMSCs after regulating SOX11; (F) ARS of hBMSCs after regulating SOX11; (G) ALP staining test of ALP activity; (H–I) qRT-PCR and western blotting assays of RUNX2 and Osterix after regulating SOX11. N = 3. Note: OM, osteogenic medium; AST, astragalus; oe, overexpression; NC, negative control; ARS, Alizarin Red S; ALP, alkaline phosphatase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
-
To further investigate the effect of AST on OP, a rat model of OP was established using OVX. Micro-CT scanning revealed that the BMD, BV/TV, Tb.N, and Tb. Th were reduced in rats with OVX, whereas gavage with AST dose-dependently increased these parameters (Figure 6A–E). HE staining showed that the femoral trabecular structure of rats with OVX was irregularly arranged, with fewer intertrabecular connections. Treatment with AST significantly increased trabecular bone volume and tightly packed trabecular bone, and significantly reduced trabecular meshwork loss (Figure 6F). Masson’s trichrome staining showed that the collagen in the OVX group was light-colored, with sparse staining, whereas in the AST group, collagen staining was deep and uniform, and the collagen fiber bundles were more closely arranged (Figure 6G). IHC staining revealed that RUNX2 expression levels significantly increased in rats with OVX after AST gavage (Figure 6H).
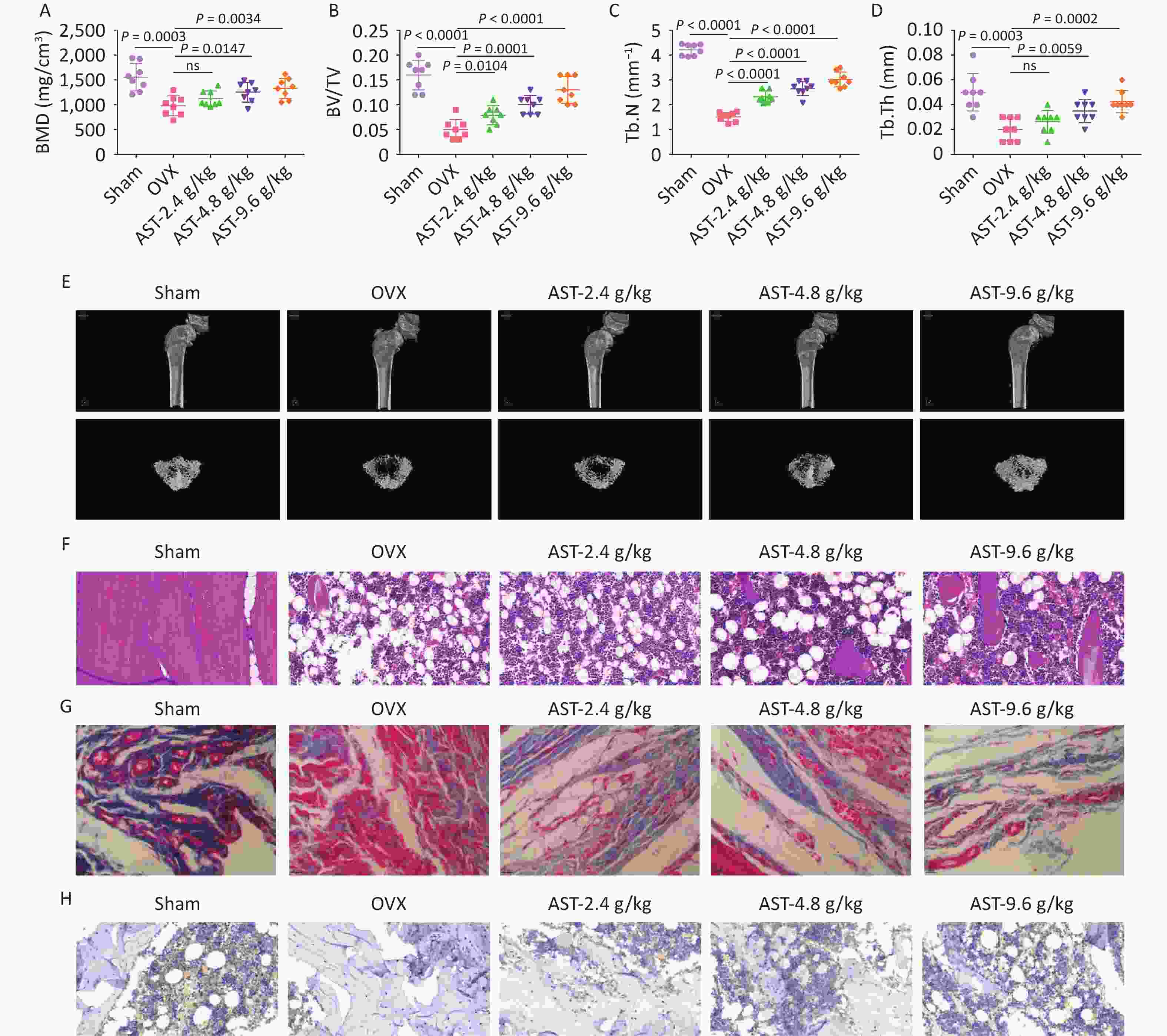
Figure 6. AST Suppresses OP in Rats. (A–E) Micro-CT scanning detection of rat BMD, BV/TV, Tb.N, and Tb.Th; (F) HE staining of the pathological conditions of bone tissue; (G) Representative Masson’s trichrome staining images of the coronal surface of the femoral head; H. IHC detection of OCN in bone tissue. N = 8. CT, computed tomography; OVX, ovariectomy; AST, astragalus; OP, osteoporosis; BMD, bone mineral density; BV/TV, bone volume to total volume ratio; Tb.N, trabecular bone number; Tb. Th, trabecular bone thickness; HE, hematoxylin and eosin; IHC, immunohistochemical.
-
After high-dose AST treatment, agomir NC, miR-181d-5p agomir, and sh-NC/-SOX11 were injected into rats, and the changes in miR-181d-5p and SOX11 levels were investigated (Figure 7A, B). Micro-CT scanning revealed that, in AST-treated rats, elevating the miR-181d-5p level or suppressing SOX11 levels reduced BMD, BV/TV, Tb.N, and Tb.Th (Figure 7C–G), exacerbated bone histopathology, and reduced RUNX2 expression levels (Figure 7H–J).
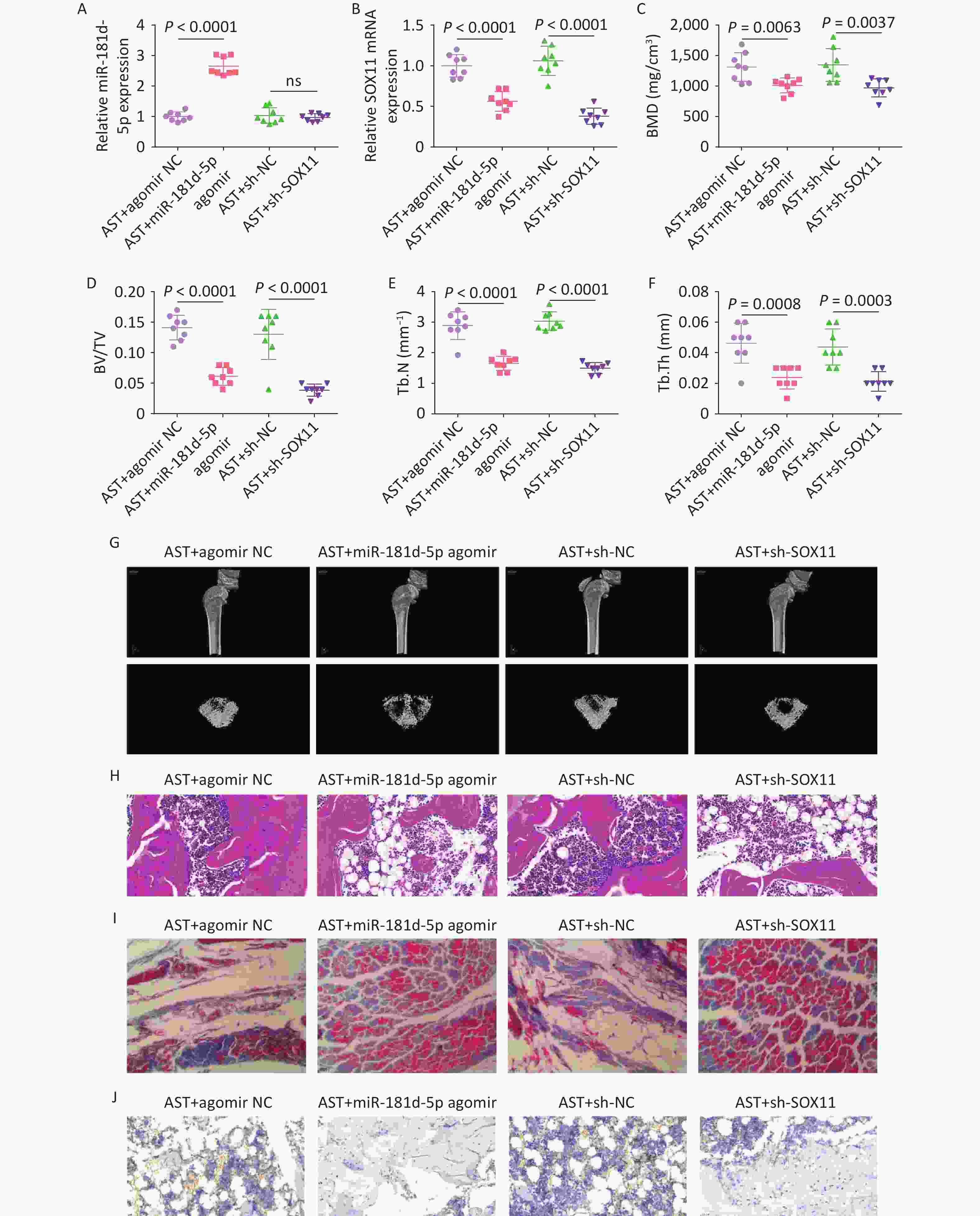
Figure 7. Increased miR-181d-5p levels or suppression of SOX11 reverses the therapeutic effect of AST on OP in rats. (A–B) qRT-PCR detection of miR-181d-5p and SOX11 in rats in vivo; (C–G) Micro-CT scan detection of BMD, BV/TV, Tb.N, Tb.Th; (H) HE staining of bone tissue; (I) Representative images of Masson’s trichrome staining of the coronal surface of the femoral head; (J) IHC detection of RUNX2 in bone tissue. N = 8. OVX, ovariectomy; AST, astragalus; NC, negative control; sh, short hairpin RNA; BMD, bone mineral density; BV/TV, bone volume to total volume ratio; Tb.N, trabecular bone number; Tb. Th, trabecular bone thickness.
-
OP is a major health problem globally. AST is a commonly used traditional medicinal that enhances immunity[35]. Emerging evidence suggests that AST alleviates the development of OP[9]. In this study, hBMSCs were induced to achieve OD in vitro, and AST treatment increased the OD of hBMSCs. AST treatment effectively increased BMD, BV/TV, Tb.N, and Tb.Th in a rat model, suggesting that AST prevents OVX-induced bone loss. These data suggested that AM treatment mitigated OP by stimulating the OD of hBMSCs, and this effect was associated with the miR-181d-5p/SOX11 axis.
AST has a protective effect against OP in rats with OVX. AST treatment increases bone mineral density and regulates bone metabolism in rats with OVX[36]. AST has also been shown to alleviate OP in rats with OVX by stimulating bone formation and modulating bone resorption[37]. Meanwhile, components of AST have also been shown to promote the OD of hBMSCs[38]. We also confirmed that AST treatment increased the viability of hBMSCs, reduced the number of G0/G1 phase cells, increased the OD of hBMSCs, and repressed apoptosis. In general, because of the striking resemblance of skeletal responses in humans and rats to a state of estrogen deficiency, rats with OVX are considered the gold standard model for evaluating drugs for the prevention and reversal of OP[39]. OVX is associated with bone turnover, negative bone balance, and loss of bone mineral density, resulting in bone loss[40]. Based on its porosity and unit microstructure, bone is generally classified as dense (cortical or compact) or trabecular (cancellous or spongy)[41]. OVX can reduce BMD, BV/TV, Tb.N, and Tb.Th in rats, such that the structure of the femoral trabecular bone is irregularly arranged, the connection between trabecular bones is less, and the collagen content is reduced[42]. These changes were also observed in OVX-treated rats and AST treatment impaired the effects of OVX. AST treatment reduced miR-181d-5p expression levels in a concentration-dependent manner, suggesting that miR-181d-5p is a downstream molecule of AST.
It has been shown that multiple miRNAs mediate osteogenesis by affecting multiple signaling pathways, play an important role in bone metabolism and OP, and can be used as potential therapeutic targets for OP treatment[43]. For example, an increase in miR-Let-7a-5p levels mitigates OP development and promotes osteoblast differentiation[13]. miR-187-5p expression levels are elevated during the OD of mouse BMSCs and this promotes their OD. Here, miR-181d-5p levels were found to be reduced during the OD of hBMSCs, and increasing miR-181d-5p levels impaired the effect of AST on hBMSCs in vitro and in OVX-treated rats.
SOX11 is a member of the SOXC family[44]. It can mediate the behavior of progenitor and stem cells and it often regulates neurogenesis and osteogenesis, along with SOX4 and SOX12[45]. SOX11 dysregulation has been observed in central nervous system developmental disorders[46], cancer[47], optic neuropathy[48], and osteoarthritis[49]. SOX11 participates in the self-renewal, OD[50], and chondrogenesis[51] of BMSCs. Moreover, SOX11 expression is regulated by miRNAs[52]. Here, we confirmed that SOX11 expression was elevated during the OD of hBMSCs. SOX11 was confirmed to be a downstream target of miR-181d-5p.
However, this study has some limitations. First, we only verified the role of AST in OP induced by estrogen deficiency and we did not further explore the role of AST in OP induced by other factors. Second, we did not further explore the downstream regulatory mechanism of SOX11, and the sample size of the animal experiments in the present study was relatively small. Moreover, the findings of this study cannot be generalized to the clinic. In future studies, we will aim to validate the role and mechanism of AST in OP induced by chronic disease or aging and confirm the results using larger and more diverse animal studies.
-
Overall, the findings of this study demonstrated that AST has an osteoprotective effect on estrogen-deficiency-induced bone loss in rats. The molecular mechanism by which AST promotes the OD of hBMSCs via the miR-181d-5p/SOX11 axis was revealed. This study provides a new approach for the treatment of OP and lays the foundation for the clinical application of AST.
doi: 10.3967/bes2025.115
Astragalus Promotes Osteogenic Differentiation of hBMSCs and Alleviates Osteoporosis by Targeting SOX11 Via mRr-181d-5p
-
Abstract:
Objective This study aimed to investigate the effect of Astragalus (AST) on osteoporosis (OP) and the downstream mechanisms. Methods Human bone marrow-derived mesenchymal stem cells (hBMSCs) were induced to differentiate into osteogenic cells. After transfection with relevant plasmids, cell proliferation, cell cycle progression, and apoptosis were assessed. Alizarin red staining was used to detect calcium nodules in the cells, alkaline phosphatase (ALP) staining was used to detect ALP activity in the cells, and quantitative reverse transcription-polymerase chain reaction and western blotting were used to determine RUNX2 and Osterix expression levels. An OP rat model was established using ovariectomy and micro-computed tomography scanning. Hematoxylin and eosin staining and Masson’s trichrome staining were used to evaluate the pathological conditions of bone tissues, while immunohistochemistry was conducted to detect RUNX2 in bone tissues. Results AST promoted the osteogenic differentiation of BMSCs, reduced miR-181d-5p expression levels, and increased SOX11 expression levels. Restoring miR-181d-5p expression or reducing SOX11 expression levels reversed the effects of AST on the osteogenic differentiation of hBMSCs. miR-181d-5p was found to target SOX11 in hBMSCs. AST improved OP in rats, and miR-181d-5p overexpression or SOX11 inhibition reversed the therapeutic effects of AST on OP in rats. Conclusion AST promoted the osteogenic differentiation of hBMSCs and alleviated OP by targeting SOX11 via miR-181d-5p. -
Key words:
- Astragalus /
- MicroRNA-181d-5p /
- SOX11 /
- Osteogenic differentiation /
- Osteoporosis
The authors have no conflicts of interest to declare.
All animal procedures and care were approved by the College of Pharmacy, Jinan University Laboratory Animal Care Committee and were performed according to NIH guidelines (ethics approval number 2018 No. [03BV]).
注释:1) Authors’ Contributions: 2) Competing Interests: 3) Ethics: -
Figure 1. AST promotes the OD of BMSCs and reduces miR-181d-5p levels in vitro. (A) 3-MTT assay for the detection of hBMSC viability; (B–C) Flow cytometry test of the cell cycle and apoptosis; (D) ARS staining of hBMSCs; (E) ALP staining test of ALP activity; (F–G) qRT-PCR and western blotting assays of RUNX2 and Osterix expression; (H) qRT-PCR analysis of miR-181d-5p levels. N = 3. OD, osteogenic differentiation; MTT, 4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; OM, osteogenic medium; hBMSC, human bone marrow-derived mesenchymal stem cell; AST, astragalus; ARS, Alizarin Red S; ALP, alkaline phosphatase; qRT-PCR, quantitative reverse transcription polymerase chain reaction; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Figure 2. Increased miR-181d-5p levels reduce the promoting effect of AST on the OD of hBMSCs. (A) qRT-PCR analysis of miR-181d-5p after regulating miR-181d-5p levels; (B) MTT assay for the detection of hBMSC viability after regulating miR-181d-5p levels; (C–D) Flow cytometry detection of the cell cycle stage and apoptosis after regulating miR-181d-5p levels; (E) ARS of hBMSCs after regulating miR-181d-5p levels; (F) ALP staining test of ALP activity; (G–H) qRT-PCR and western blotting assays of RUNX2 and Osterix after regulating miR-181d-5p levels. N = 3. OM, osteogenic medium; AST, astragalus; NC, negative control; in, inhibitor; ARS, Alizarin Red S; ALP, alkaline phosphatase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Figure 3. miR-181d-5p Targets SOX11 In hBMSCs. (A–D) qRT-PCR and western blotting detection of SOX11 in hBMSCs; (E) Bioinformatics website prediction of the binding site of miR-181d-5p and SOX11; (F) Luciferase activity assay to verify the relationship between miR-181d-5p and SOX11; (G) RIP assay to confirm the binding of miR-181d-5p to SOX11. N = 3. OM, osteogenic medium; AST, astragalus; NC, negative control; in, inhibitor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; wt, wild-type; mut, mutant; ns, not significant; RIP, RNA immunoprecipitation.
Figure 4. SOX11 Further Promotes the Effect of AST on the OD of hBMSCs. (A–B) qRT-PCR and western blotting detection of SOX11 after regulating SOX11; (C) MTT detection of hBMSC viability after regulating SOX11; (D–E) Flow cytometry detection of the cell cycle stage and apoptosis after regulating SOX11; (F) ARS of hBMSCs after regulating SOX11; (G) ALP staining test of ALP activity; (H–I) qRT-PCR and Western blotting assays of RUNX2 and Osterix after SOX11 regulation. N = 3. OM, osteogenic medium; AST, astragalus; sh, short hairpin RNA; NC, negative control; oe, overexpression; ARS, Alizarin Red S; ALP, alkaline phosphatase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Figure 5. SOX11 reversed the inhibition of hBMSCs OD mediated by miR-181d-5p. (A–B) qRT-PCR and western blotting detection of SOX11 after regulating SOX11; (C) MTT detection of hBMSCs cell viability after regulating SOX11; (D–E) Flow cytometry detection of the cell cycle stage and apoptosis of hBMSCs after regulating SOX11; (F) ARS of hBMSCs after regulating SOX11; (G) ALP staining test of ALP activity; (H–I) qRT-PCR and western blotting assays of RUNX2 and Osterix after regulating SOX11. N = 3. Note: OM, osteogenic medium; AST, astragalus; oe, overexpression; NC, negative control; ARS, Alizarin Red S; ALP, alkaline phosphatase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Figure 6. AST Suppresses OP in Rats. (A–E) Micro-CT scanning detection of rat BMD, BV/TV, Tb.N, and Tb.Th; (F) HE staining of the pathological conditions of bone tissue; (G) Representative Masson’s trichrome staining images of the coronal surface of the femoral head; H. IHC detection of OCN in bone tissue. N = 8. CT, computed tomography; OVX, ovariectomy; AST, astragalus; OP, osteoporosis; BMD, bone mineral density; BV/TV, bone volume to total volume ratio; Tb.N, trabecular bone number; Tb. Th, trabecular bone thickness; HE, hematoxylin and eosin; IHC, immunohistochemical.
Figure 7. Increased miR-181d-5p levels or suppression of SOX11 reverses the therapeutic effect of AST on OP in rats. (A–B) qRT-PCR detection of miR-181d-5p and SOX11 in rats in vivo; (C–G) Micro-CT scan detection of BMD, BV/TV, Tb.N, Tb.Th; (H) HE staining of bone tissue; (I) Representative images of Masson’s trichrome staining of the coronal surface of the femoral head; (J) IHC detection of RUNX2 in bone tissue. N = 8. OVX, ovariectomy; AST, astragalus; NC, negative control; sh, short hairpin RNA; BMD, bone mineral density; BV/TV, bone volume to total volume ratio; Tb.N, trabecular bone number; Tb. Th, trabecular bone thickness.
Table 1. Primer sequence
Genes Primer sequence (5'-3') miR-181d-5p Human F: 5'-GGCAGAACATTCATTGTTGTCG-3' R: 5'-GCAGGGTCCGAGGTATTC-3' Rat F: 5'-GGCAGAACATTCATTGTTGTCG-3' R: 5'-GCAGGGTCCGAGGTATTC-3' U6 Human F: 5'-CTCGCTTCGGCAGCACA-3' R: 5'-AACGCTTCACGAATTTGCGT-3' Rat F: 5'-CTCGCTTCGGCAGCACA-3' R: 5'-AACGCTTCACGAATTTGCGT-3' SOX11 Human F: 5'-CCAGGACAGAACCACCTGAT-3' R: 5'-CCCCACAAACCACTCAGACT-3' Rat F: 5'-CAGCGAGAAGATCCCGTTCA-3' R: 5'-GTCCGTCTTGGGCTTTTTGC-3' RUNX2 Human F: 5'-GCGCATTCCTCATCCCAGTA-3' R: 5'-GGCTCAGGTAGGAGGGGTAA-3' Osterix Human F: 5'-GATGGCGTCCTCTCTGCTTG-3' R: 5'-AATGGGCTTCTTCCTCAGCC-3' GAPDH Human F: 5'-CACCCACTCCTCCACCTTTG-3' R: 5'-CCACCACCCTGTTGCTGTAG-3' Rat F: 5'-GTCGGTGTGAACGGATTTG-3' R: 5'-TCCCATTCTCAGCCTTGAC-3' Note. F, forward; R, reverse; U6, U6 small nuclear RNA; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. -
[1] Gamsjaeger S, Fratzl P, Paschalis EP. Interplay between mineral crystallinity and mineral accumulation in health and postmenopausal osteoporosis. Acta Biomater, 2021; 124, 374−81. doi: 10.1016/j.actbio.2021.02.011 [2] Zhang L, Zheng YL, Wang R, et al. Exercise for osteoporosis: a literature review of pathology and mechanism. Front Immunol, 2022; 13, 1005665. doi: 10.3389/fimmu.2022.1005665 [3] Luo M, Zhao ZH, Yi JR. Osteogenesis of bone marrow mesenchymal stem cell in hyperglycemia. Front Endocrinol (Lausanne), 2023; 14, 1150068. doi: 10.3389/fendo.2023.1150068 [4] Mohamed-Ahmed S, Fristad I, Lie SA, et al. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res Ther, 2018; 9, 168. doi: 10.1186/s13287-018-0914-1 [5] Chu DT, Phuong TNT, Tien NLB, et al. An update on the progress of isolation, culture, storage, and clinical application of human bone marrow mesenchymal stem/stromal cells. Int J Mol Sci, 2020; 21, 708. doi: 10.3390/ijms21030708 [6] Wang C, Meng HY, Wang X, et al. Differentiation of bone marrow mesenchymal stem cells in osteoblasts and adipocytes and its role in treatment of osteoporosis. Med Sci Monit, 2016; 22, 226−33. doi: 10.12659/MSM.897044 [7] Wang LL, Xiong F, Yang LC, et al. A seasonal change of active ingredients and mineral elements in root of Astragalus membranaceus in the Qinghai-Tibet Plateau. Biol Trace Elem Res, 2021; 199, 3950−9. doi: 10.1007/s12011-020-02486-0 [8] Chai YH, Pu X, Wu YZ, et al. Inhibitory effect of Astragalus Membranaceus on osteoporosis in SAMP6 mice by regulating vitaminD/FGF23/Klotho signaling pathway. Bioengineered, 2021; 12, 4464−74. doi: 10.1080/21655979.2021.1946633 [9] Jung Koo H, Sohn EH, Kim YJ, et al. Effect of the combinatory mixture of Rubus coreanus Miquel and Astragalus membranaceus Bunge extracts on ovariectomy-induced osteoporosis in mice and anti-RANK signaling effect. J Ethnopharmacol, 2014; 151, 951−9. doi: 10.1016/j.jep.2013.12.008 [10] He YH, Chen D, Guo Q, et al. MicroRNA-151a-3p functions in the regulation of osteoclast differentiation: significance to postmenopausal osteoporosis. Clin Interv Aging, 2021; 16, 1357−66. doi: 10.2147/CIA.S289613 [11] Ge DW, Wang WW, Chen HT, et al. Functions of microRNAs in osteoporosis. Eur Rev Med Pharmacol Sci, 2017; 21, 4784−9. [12] Zhang J, Zhang T, Tang BS, et al. The miR-187 induced bone reconstruction and healing in a mouse model of osteoporosis, and accelerated osteoblastic differentiation of human multipotent stromal cells by targeting BARX2. Pathol Res Pract, 2021; 219, 153340. doi: 10.1016/j.prp.2021.153340 [13] Alrashed MM, Alshehry AS, Ahmad M, et al. miRNA Let-7a-5p targets RNA KCNQ1OT1 and participates in osteoblast differentiation to improve the development of osteoporosis. Biochem Genet, 2022; 60, 370−81. doi: 10.1007/s10528-021-10105-3 [14] Tang XY, Li XY, Zhang DY, et al. Astragaloside-IV alleviates high glucose-induced ferroptosis in retinal pigment epithelial cells by disrupting the expression of miR-138-5p/Sirt1/Nrf2. Bioengineered, 2022; 13, 8238−53. doi: 10.1080/21655979.2022.2049471 [15] Wei YC, Wu Y, Feng K, et al. Astragaloside IV inhibits cardiac fibrosis via miR-135a-TRPM7-TGF-β/Smads pathway. J Ethnopharmacol, 2020; 249, 112404. doi: 10.1016/j.jep.2019.112404 [16] Li DX, Li GC, Chen Y, et al. Astragaloside IV protects ATDC5 cells from lipopolysaccharide-caused damage through regulating miR-203/MyD88. Pharm Biol, 2020; 58, 89−97. doi: 10.1080/13880209.2019.1705355 [17] Cao YJ, Lv QX, Li Y. Astragaloside IV improves tibial defect in rats and promotes proliferation and osteogenic differentiation of hBMSCs through MiR-124-3p. 1/STAT3 axis. J Nat Prod, 2021; 84, 287−97. doi: 10.1021/acs.jnatprod.0c00975 [18] Wu XH, Dou B, Sun NY, et al. Astragalus saponin IV promotes osteogenic differentiation of bone marrow mesenchymal stem cells via miR-21/NGF/BMP2/Runx2 pathway. Acta Histochem, 2020; 122, 151549. doi: 10.1016/j.acthis.2020.151549 [19] Waki T, Lee SY, Niikura T, et al. Profiling microRNA expression during fracture healing. BMC Musculoskelet Disord, 2016; 17, 83. doi: 10.1186/s12891-016-0931-0 [20] Liu YP, Wang YX, Cheng X, et al. MiR-181d-5p regulates implant surface roughness-induced osteogenic differentiation of bone marrow stem cells. Mater Sci Eng C Mater Biol Appl, 2021; 121, 111801. doi: 10.1016/j.msec.2020.111801 [21] Dai YT, Wang DB, Zhao MJ, et al. Quality markers for astragali radix and its products based on process analysis. Front Pharmacol, 2020; 11, 554777. doi: 10.3389/fphar.2020.554777 [22] Li P, Kong JC, Chen ZM, et al. Aloin promotes osteogenesis of bone-marrow-derived mesenchymal stem cells via the ERK1/2-dependent Runx2 signaling pathway. J Nat Med, 2019; 73, 104−13. doi: 10.1007/s11418-018-1249-z [23] Shen WX, Sun B, Zhou CH, et al. CircFOXP1/FOXP1 promotes osteogenic differentiation in adipose-derived mesenchymal stem cells and bone regeneration in osteoporosis via miR-33a-5p. J Cell Mol Med, 2020; 24, 12513−24. doi: 10.1111/jcmm.15792 [24] Ren LR, Yao RB, Wang SY, et al. MiR-27a-3p promotes the osteogenic differentiation by activating CRY2/ERK1/2 axis. Mol Med, 2021; 27, 43. [25] Zhong LN, Zhang YZ, Li H, et al. Overexpressed miR-196a accelerates osteogenic differentiation in osteoporotic mice via GNAS-dependent Hedgehog signaling pathway. J Cell Biochem, 2019; 120, 19422−31. doi: 10.1002/jcb.29166 [26] Liu H, Yi X, Tu ST, et al. Kaempferol promotes BMSC osteogenic differentiation and improves osteoporosis by downregulating miR-10a-3p and upregulating CXCL12. Mol Cell Endocrinol, 2021; 520, 111074. doi: 10.1016/j.mce.2020.111074 [27] Huang Q, Zhang F, Fu HY, et al. Epigenetic regulation of miR-518a-5p-CCR6 feedback loop promotes both proliferation and invasion in diffuse large B cell lymphoma. Epigenetics, 2021; 16, 28−44. doi: 10.1080/15592294.2020.1786317 [28] You MR, Zhang L, Zhang XX, et al. MicroRNA-197-3p inhibits the osteogenic differentiation in osteoporosis by down-regulating KLF 10. Clin Interv Aging, 2021; 16, 107−17. doi: 10.2147/CIA.S269171 [29] Che MX, Gong WQ, Zhao Y, et al. Long noncoding RNA HCG18 inhibits the differentiation of human bone marrow-derived mesenchymal stem cells in osteoporosis by targeting miR-30a-5p/NOTCH1 axis. Mol Med, 2020; 26, 106. [30] Li T, Jiang HX, Li Y, et al. Estrogen promotes lncRNA H19 expression to regulate osteogenic differentiation of BMSCs and reduce osteoporosis via miR-532-3p/SIRT1 axis. Mol Cell Endocrinol, 2021; 527, 111171. doi: 10.1016/j.mce.2021.111171 [31] Lu JS, Yang JZ, Zheng YS, et al. Extracellular vesicles from endothelial progenitor cells prevent steroid-induced osteoporosis by suppressing the ferroptotic pathway in mouse osteoblasts based on bioinformatics evidence. Sci Rep, 2019; 9, 16130. doi: 10.1038/s41598-019-52513-x [32] Wang QJ, Li Y, Zhang YX, et al. LncRNA MEG3 inhibited osteogenic differentiation of bone marrow mesenchymal stem cells from postmenopausal osteoporosis by targeting miR-133a-3p. Biomed Pharmacother, 2017; 89, 1178−86. doi: 10.1016/j.biopha.2017.02.090 [33] Sun JR, Zhang X, Zhang Y. MiR-214 prevents the progression of diffuse large B-cell lymphoma by targeting PD-L1. Cell Mol Biol Lett, 2019; 24, 68. doi: 10.1186/s11658-019-0190-9 [34] Lin C, Zhong WJ, Yan W, et al. Circ-SLC8A1 regulates osteoporosis through blocking the inhibitory effect of miR-516b-5p on AKAP2 expression. J Gene Med, 2020; 22, e3263. doi: 10.1002/jgm.3263 [35] Li YG, Yu P, Fu WW, et al. Ginseng-Astragalus-oxymatrine injection ameliorates cyclophosphamide-induced immunosuppression in mice and enhances the immune activity of RAW264.7 cells. J Ethnopharmacol, 2021; 279, 114387. doi: 10.1016/j.jep.2021.114387 [36] Kang SC, Kim HJ, Kim MH. Effects of Astragalus membranaceus with supplemental calcium on bone mineral density and bone metabolism in calcium-deficient ovariectomized rats. Biol Trace Elem Res, 2013; 151, 68−74. doi: 10.1007/s12011-012-9527-1 [37] Huh JE, Kim SJ, Kang JW, et al. The standardized BHH10 extract, a combination of Astragalus membranaceus, Cinnamomum cassia, and Phellodendron amurense, reverses bone mass and metabolism in a rat model of postmenopausal osteoporosis. Phytother Res, 2015; 29, 30−9. doi: 10.1002/ptr.5218 [38] Sun NY, Liu XL, Gao J, et al. Astragaloside-IV modulates NGF-induced osteoblast differentiation via the GSK3β/β-catenin signalling pathway. Mol Med Rep, 2021; 23, 19. [39] Kharode YP, Sharp MC, Bodine PVN. Utility of the ovariectomized rat as a model for human osteoporosis in drug discovery. Methods Mol Biol, 2008; 455, 111−24. [40] Liu F, Yuan YJ, Bai L, et al. LRRc17 controls BMSC senescence via mitophagy and inhibits the therapeutic effect of BMSCs on ovariectomy-induced bone loss. Redox Biol, 2021; 43, 101963. doi: 10.1016/j.redox.2021.101963 [41] Yeh PS, Lee YW, Chang WH, et al. Biomechanical and tomographic differences in the microarchitecture and strength of trabecular and cortical bone in the early stage of male osteoporosis. PLoS One, 2019; 14, e0219718. doi: 10.1371/journal.pone.0219718 [42] Zhu MS, Shan J, Xu HE, et al. Glaucocalyxin A suppresses osteoclastogenesis induced by RANKL and osteoporosis induced by ovariectomy by inhibiting the NF-κB and Akt pathways. J Ethnopharmacol, 2021; 276, 114176. doi: 10.1016/j.jep.2021.114176 [43] De-Ugarte L, Yoskovitz G, Balcells S, et al. MiRNA profiling of whole trabecular bone: identification of osteoporosis-related changes in MiRNAs in human hip bones. BMC Med Genomics, 2015; 8, 75. [44] Oliemuller E, Newman R, Tsang SM, et al. SOX11 promotes epithelial/mesenchymal hybrid state and alters tropism of invasive breast cancer cells. eLife, 2020; 9, e58374. doi: 10.7554/eLife.58374 [45] Tsang SM, Oliemuller E, Howard BA. Regulatory roles for SOX11 in development, stem cells and cancer. Semin Cancer Biol, 2020; 67, 3−11. doi: 10.1016/j.semcancer.2020.06.015 [46] Kavyanifar A, Turan S, Lie DC. SoxC transcription factors: multifunctional regulators of neurodevelopment. Cell Tissue Res, 2018; 371, 91−103. doi: 10.1007/s00441-017-2708-7 [47] Wasik AM, Lord M, Wang X, et al. SOXC transcription factors in mantle cell lymphoma: the role of promoter methylation in SOX11 expression. Sci Rep, 2013; 3, 1400. doi: 10.1038/srep01400 [48] Chang KC, Hertz J. SoxC transcription factors in retinal development and regeneration. Neural Regen Res, 2017; 12, 1048−51. doi: 10.4103/1673-5374.211178 [49] Xu SP, Yu JC, Wang ZR, et al. SOX11 promotes osteoarthritis through induction of TNF-α. Pathol Res Pract, 2019; 215, 152442. doi: 10.1016/j.prp.2019.152442 [50] Yu FY, Wu FZ, Li FF, et al. Wnt7b-induced Sox11 functions enhance self-renewal and osteogenic commitment of bone marrow mesenchymal stem cells. Stem Cells, 2020; 38, 1020−33. doi: 10.1002/stem.3192 [51] Kato K, Bhattaram P, Penzo-Méndez A, et al. SOXC transcription factors induce cartilage growth plate formation in mouse embryos by promoting noncanonical WNT signaling. J Bone Miner Res, 2015; 30, 1560−71. doi: 10.1002/jbmr.2504 [52] Meng CY, Xue F, Zhao ZQ, et al. Influence of MicroRNA-141 on inhibition of the proliferation of bone marrow mesenchymal stem cells in steroid-induced osteonecrosis via SOX11. Orthop Surg, 2020; 12, 277−85. doi: 10.1111/os.12603 -




 下载:
下载:




 Quick Links
Quick Links