-
The escalating prevalence of genitourinary system diseases (GUDs), fueled by lifestyle changes and aging populations, represents an increasingly significant yet often overlooked global health challenge[1,2]. Kidney diseases alone impact over 8.5 million people worldwide[3], making them the third fastest-growing cause of mortality[4], with a particularly heavy burden in developing countries[5]. Urinary tract infections (UTIs), among the most widespread infectious diseases, result in over 150 million cases annually[6]. Chronic kidney disease (CKD) underscores this crisis further, with 697.5 million cases and 1.2 million deaths reported in 2017—marking a 41.5% increase since 1990[5]. Notably, kidney dysfunction independently heightens cardiovascular risks, especially in hypertensive and diabetic individuals, contributing to 1.4 million cardiovascular deaths (7.6% of total cardiovascular mortality) in 2017[5,7-9]. Despite these alarming statistics, public awareness remains severely lacking; only 6% of patients with CKD are aware of their condition[10]. Addressing risk factors for GUDs whose health impacts are mitigable through adaptive interventions is therefore an urgent public health priority.
The incidence of GUDs is influenced by multiple factors, including metabolic abnormalities, chronic conditions, drug toxicity, and infections[11-13]. Growing attention has been paid in recent years to environmental risk factors, particularly those involving non-optimal temperatures, due to universal human exposure. Emerging evidence indicates that cold exposure may represent an often-overlooked environmental risk factor for GUDs, potentially surpassing the well-documented effects of heat[14]. From a mechanistic perspective, cold exposure may disrupt renal homeostasis through dehydration, hemodynamic instability, and reduced renal perfusion due to cardiovascular stress[15,16]. Furthermore, cold-induced vasoconstriction or frostbite can exacerbate tissue injury and cold exposure triggers sympathetic nervous system activation, collectively contributing to both acute and chronic kidney damage[17-19]. Several epidemiological studies have reported associations between cold exposure and an increased risk of GUDs; however, research specifically examining the link between cold spells and GUD outcomes remains scarce. As prolonged episodes of low temperatures, cold spells may not only confer risks directly attributed to low temperatures but also potentially generate additive effects from sustained cold stress. Only two studies have directly examined the impact of cold spells on GUDs: one in Guangzhou, China, which associated cold. spells with higher GUD mortality (relative risk (RR) = 1.75, 95% confidence interval (95% CI): 1.14–2.68)[20], and another nationwide analysis in Korea, which linked cold spells to increased acute kidney injury (AKI) related hospitalizations (RR = 1.87, 95% CI: 1.46–2.39) and mortality (RR = 4.84, 95% CI: 1.30–17.98)[18]. These findings suggest cold spells are a likely, yet underappreciated risk factor. However, current epidemiological evidence remains scarce.
To address these gaps, this study investigated the association between cold spells and the risk of GUD hospitalization in Beijing, China, as well as the risks for specific subtypes of GUDs, including urinary system diseases and renal failure. Additionally, subgroup risk analyzes were conducted to explore how these associations varied across admission type, sex, and age. The findings will expand the existing epidemiological evidence on cold spells and GUDs hospitalization, providing actionable insights to inform public health strategies and potentially reducing the disease burden.
-
Daily hospitalization data, meteorological records, and air pollution data for Beijing, China, were collected from January 1, 2013, to December 31, 2018. Beijing, a northern metropolis that has experienced recurrent extreme cold spells in recent years, possesses comprehensive and representative health outcome data spanning the cold season, thereby offering an ideal setting for investigation of cold spell–related health risks. Daily admission numbers for GUDs (International Statistical Classification of Diseases 10th Revision, ICD-10: N00-N99), urinary system diseases (ICD-10: N00-N39), and renal failure (ICD-10: N17-N19) across secondary and tertiary hospitals in all districts of Beijing were obtained from the Beijing Municipal Health Commission Information Center. Districts were determined based on patients' usual place of residence, defined as having lived at the address for six months or more. These hospitals accounted for approximately 95% of the total hospital bed capacity in the city[21]. The study population was categorized into seven subgroups based on admission type (emergency vs. outpatient), sex (male vs. female), and age (0-64 years, 65-74 years, and ≥ 75 years)[22].
City-level daily mean temperature and relative humidity of Beijing monitored by site 54511 were obtained from the China Meteorological Data Sharing Service (http://data.cma.cn). Daily PM2.5 concentrations of each district were provided by the Beijing Municipal Ecological and Environmental Monitoring Center.
-
Five cold spell definitions were established: p1day2, p2day2, p3day2, p4day2, and p5day2, each representing periods with a daily mean temperature below the 1st, 2nd, 3rd, 4th, and 5th percentiles of the daily mean temperature distribution from January 1, 2013, to December 31, 2018, lasting for two or more consecutive days (Supplementary Table S1). The percentile thresholds were calculated using pooled daily mean temperature data across the entire 2013–2018 period, rather than annual thresholds computed separately for each individual year. The preponderance of studies on cold spell health risks has employed relative temperature thresholds and durations to define cold spells[23-25].
-
A two-stage time series analysis was employed to investigate the association between cold spell exposure and cause-specific hospital admissions. Daily cold spell exposure (0 or 1) was matched with cause-specific hospitalization counts for each district from November to March (the cold season) between 2013 and 2018. In the first stage, a generalized linear model based on the quasi-Poisson distribution was applied to estimate the district-specific relative risks for hospital admissions on cold spell days compared to non-cold spell days for each cold spell definition and outcome:
$$\begin{aligned} logE\left({Y}_{t}\right)=& \alpha +\beta CS+ns\left(Time,4\times year\right)+ns\left(Tmean,2\right)+\\ & ns\left(RH,2\right)+Holiday+DOW \end{aligned} $$ In this model, where E(Yt) represents the conditional expectation of the daily count of hospital admissions for a specific outcome on day t in district i. CS is an indicator variable that denotes whether a day is classified as a cold spell day (denoted as 1) or a non-cold spell day (denoted as 0); A natural spline (ns) with 4 degrees of freedom per year (from November to March) was applied to control for long-term trends and seasonality in the data; A natural spline (ns) with 2 degrees of freedom was used to adjust for potential confounding effects of daily mean temperature (Tmean) and relative humidity (RH), considering their possible associations with hospitalization outcomes[23,26,27]; Holiday is an indicator variable for public holidays, and DOW is an indicator variable for the day of the week. Additionally, the lagged effect patterns of cold spells were analyzed in different single-day lags, considering lag times up to a maximum of 14 days, ranging from the present day (lag 0) to a 14-day lag (lag 14)[23].
In the second stage, we conducted a random-effects meta-analysis to pool the district-specific relative risk estimates, specifically the beta coefficients (β) and their corresponding standard errors obtained in the first stage. The meta-analysis was performed separately for each lag day (ranging from 0 to 14 days), each predefined cold spell definition and each specific health outcome. For each combination of lag day, cold spell definition and health outcome, the analysis generated an overall pooled RR measure, along with its corresponding 95% CI. All meta-analytic procedures were implemented using the “metafor” package within the R statistical software environment.
Additionally, to validate the stability of the model results, we repeated the analysis using gridded temperature data from the European Centre for Medium-Range Weather Forecasts (ECMWF). The supplementary material provides detailed information on the data sources and processing methods. Furthermore, we calculated the 14-day cumulative effects using the Distributed Lag Non-linear Model (DLNM), which is a flexible framework for modelling lagged and non-linear exposure-disease associations. The specific model specifications are detailed in the supplementary material.
-
Data were further stratified by admission type and population subgroups, categorized by admission type (emergency vs. outpatient), sex (male vs. female), and age (0−64 years, 65−74 years, and ≥ 75 years). Separate models were then applied to estimate the associations between p1day2 cold spells and cause-specific hospitalizations within these subgroups.
-
During the study period, there were 271,579 hospital admissions for GUDs, 110,412 for urinary system diseases, and 34,925 for renal failure. The average daily hospitalization for GUDs was 18.7 cases per district, with 2.3 of these being emergency admissions. Males accounted for 6.7 cases per day, approximately half the rate of females. The average daily hospitalization were 14.2 cases for the 0-64 years age group, 2.1 cases for the 65-74 years group, and 2.4 cases for those aged ≥ 75 years. Additionally, urinary system diseases accounted for an average of 7.6 daily admissions, including 2.4 cases of renal failure (Table 1).
Table 1. Daily district-level hospital admissions during the cold season (November to March) from 2013 to 2018 across the 16 districts of Beijing
Variables Mean ± SD Min P25 Median P75 Max Genitourinary system disease (N00-N99) 18.7 ± 19.7 0 5 11 26 168 Admission type Emergency admission 2.3 ± 2.5 0 0 2 3 23 Outpatient admission 16.4 ± 18.2 0 4 10 23 155 Sex Male 6.7 ± 6.9 0 2 4 9 59 Female 12 ± 13.4 0 3 7 17 109 Age 0–64 14.2 ± 15.2 0 4 9 20 124 65–74 2.1 ± 2.6 0 0 1 3 26 ≥ 75 2.4 ± 3.1 0 0 1 3 26 Urinary system disease (N00-N39) 7.6 ± 7.6 0 2 5 11 75 Admission type Emergency admission 1.4 ± 1.6 0 0 1 2 18 Outpatient admission 6.3 ± 6.6 0 2 4 9 66 Sex Male 4.3 ± 4.6 0 1 3 6 42 Female 3.3 ± 3.5 0 1 2 5 33 Age 0–64 4.5 ± 4.5 0 1 3 6 43 65–74 1.4 ± 1.7 0 0 1 2 18 ≥ 75 1.8 ± 2.4 0 0 1 2 18 Renal failure (N17-N19) 2.4 ± 2.7 0 0 1 4 19 Admission type Emergency admission 0.4 ± 0.8 0 0 0 1 7 Outpatient admission 2.0 ± 2.4 0 0 1 3 16 Sex Male 1.3 ± 1.7 0 0 1 2 12 Female 1.1 ± 1.5 0 0 1 2 12 Age 0–64 1.1 ± 1.4 0 0 1 2 11 65–74 0.5 ± 0.9 0 0 0 1 7 ≥ 75 0.8 ± 1.2 0 0 0 1 10 Note. SD, standard deviation. The daily mean temperature during the study period was 0°C, and the mean relative humidity was 44.0%. A total of 336 p1day2 cold spell days and 1520 p5day2 cold spell days were identified. The daily mean temperature on p1day2 cold spell days was −8.6°C, while on p5day2 cold spell days, it was −5.7°C (Table 2).
Table 2. Daily mean temperature during cold spell days in Beijing from 2013 to 2018
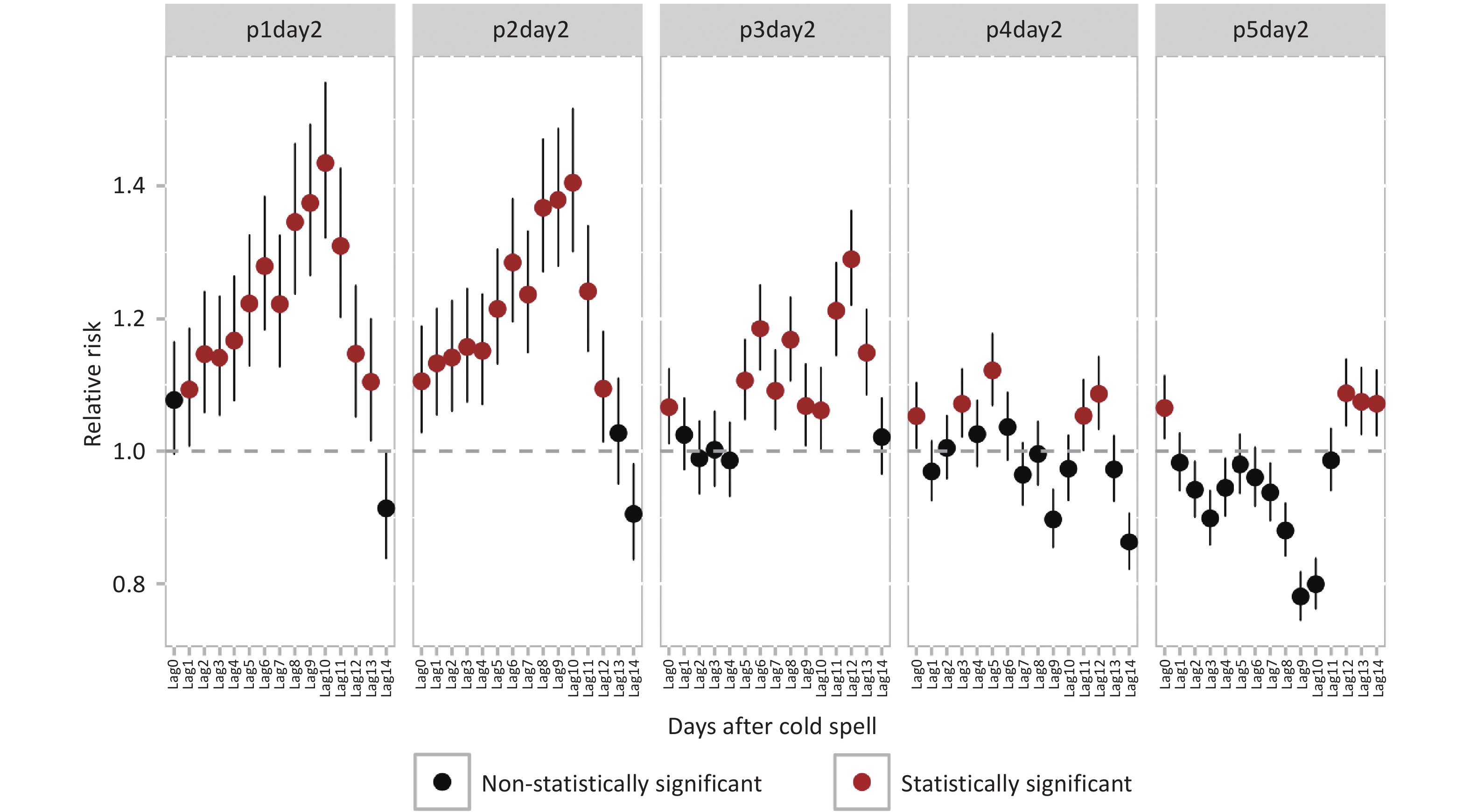
Definition Days/district, n Daily mean temperature, °C Mean ± SD Min P25 Median P75 Max p1day2 336 −8.6 ± 1.5 −14.3 −9.1 −8.1 −7.7 −7.2 p2day2 576 −7.6 ± 1.9 −14.3 −8.3 −7.5 −6.2 −5.4 p3day2 864 −6.8 ± 1.8 −14.3 −7.8 −6.1 −5.4 −4.7 p4day2 1184 −6.2 ± 1.9 −14.3 −7.4 −5.6 −4.8 −3.9 p5day2 1520 −5.7 ± 1.9 −14.3 −6.8 −5.2 −4.3 −3.3 Note. SD, standard deviation. Cold spell exposure, across all five definitions, was significantly associated with increased hospital admissions for GUDs, though the lag effects varied (Figure 1). Risk was notably elevated with p1day2 cold spell exposure at lag1, whereas for other cold spell intensities, the increase in risk was observed on the day of exposure. The highest RRs occurred at different lags: p1day2 and p2day2 at lag10, p3day2 and p5day2 at lag12, and p4day2 at lag5. Compared to non-cold spell days, the RRs for GUDs on p1day2 cold spell days at lag10 was 1.43 (95% CI: 1.32–1.56), the highest among the five cold spell definitions.
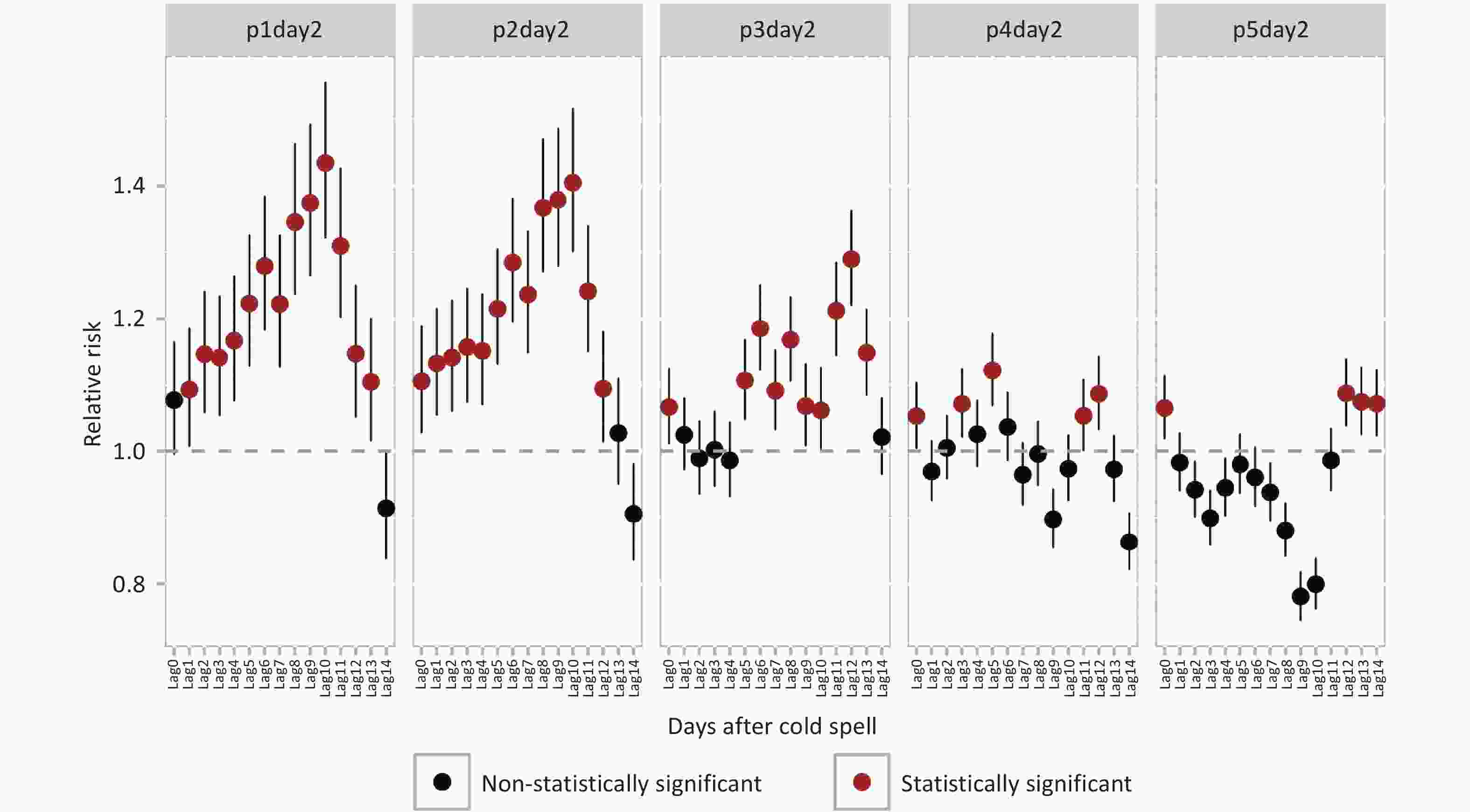
Figure 1. Relative risks and 95% confidence intervals for genitourinary system diseases hospital admissions associated with intensity-specific cold spells on different lag days.
Sensitivity analyses (Supplementary Table S2) showed that estimates from the main model remained consistent when varying the degrees of freedom for time and temperature or adjusting for relative humidity. Furthermore, analyses based on alternative data sources and models (Supplementary Figures S1 and S2) produced results that showed consistent trends.
Hospital admissions for both urinary system diseases and renal failure showed significant increases after exposure to p1day2 cold spells. For urinary system disease, the risk began to rise significantly from lag3, while for renal failure, the increase in risk was significant starting from lag7. The highest RRs for both urinary system disease and renal failure hospital admissions were observed at lag10. Compared to non-cold spell days, the RR for renal failure hospital admissions at lag10 was 1.46 (95% CI: 1.28–1.67). This was higher than the RR for urinary system diseases (RR = 1.35, 95% CI: 1.23–1.49) at the same lag (Figure 2).
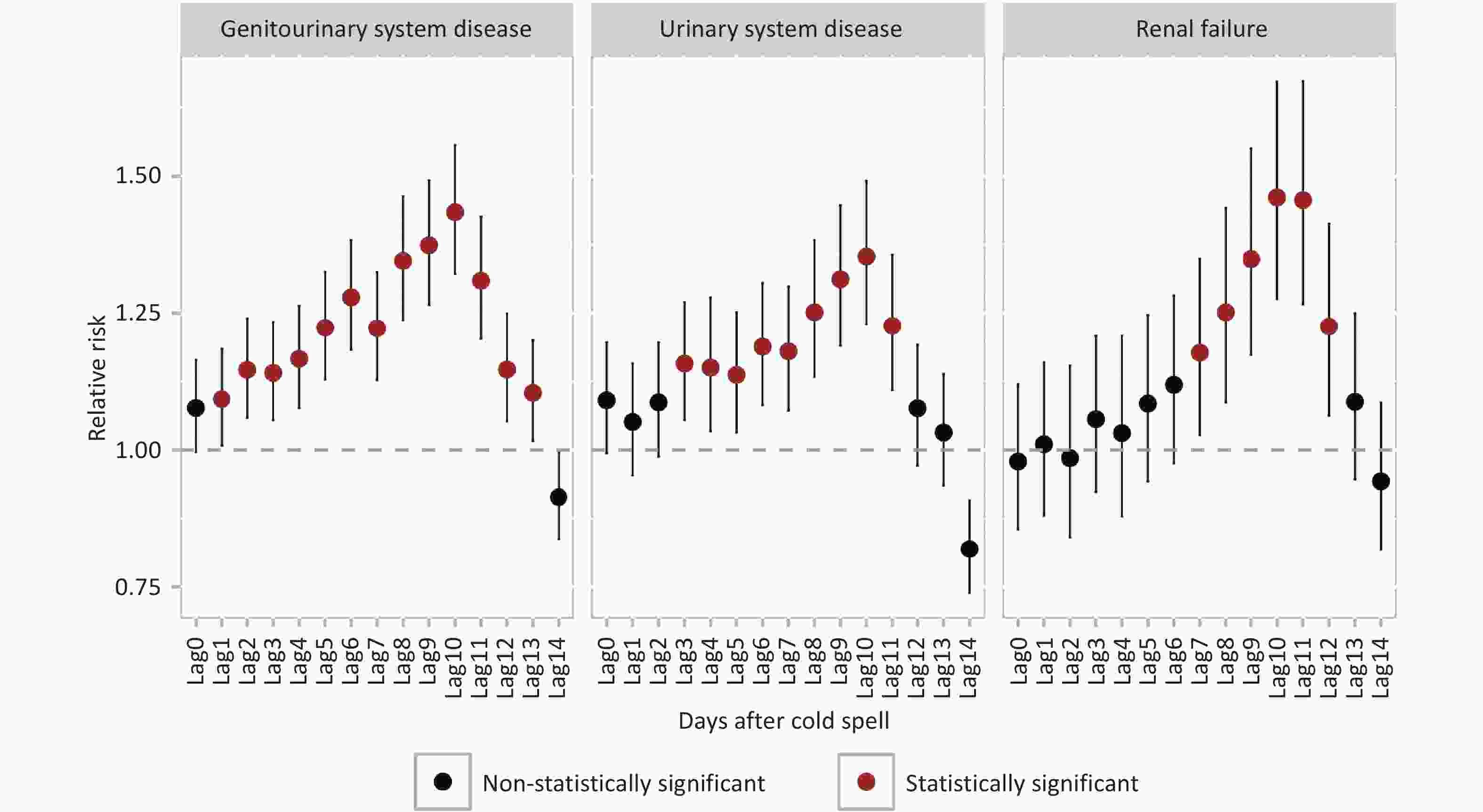
Figure 2. Relative risks and 95% confidence intervals for three cause-specific diseases associated with p1day2 cold spell exposure on different lag days.
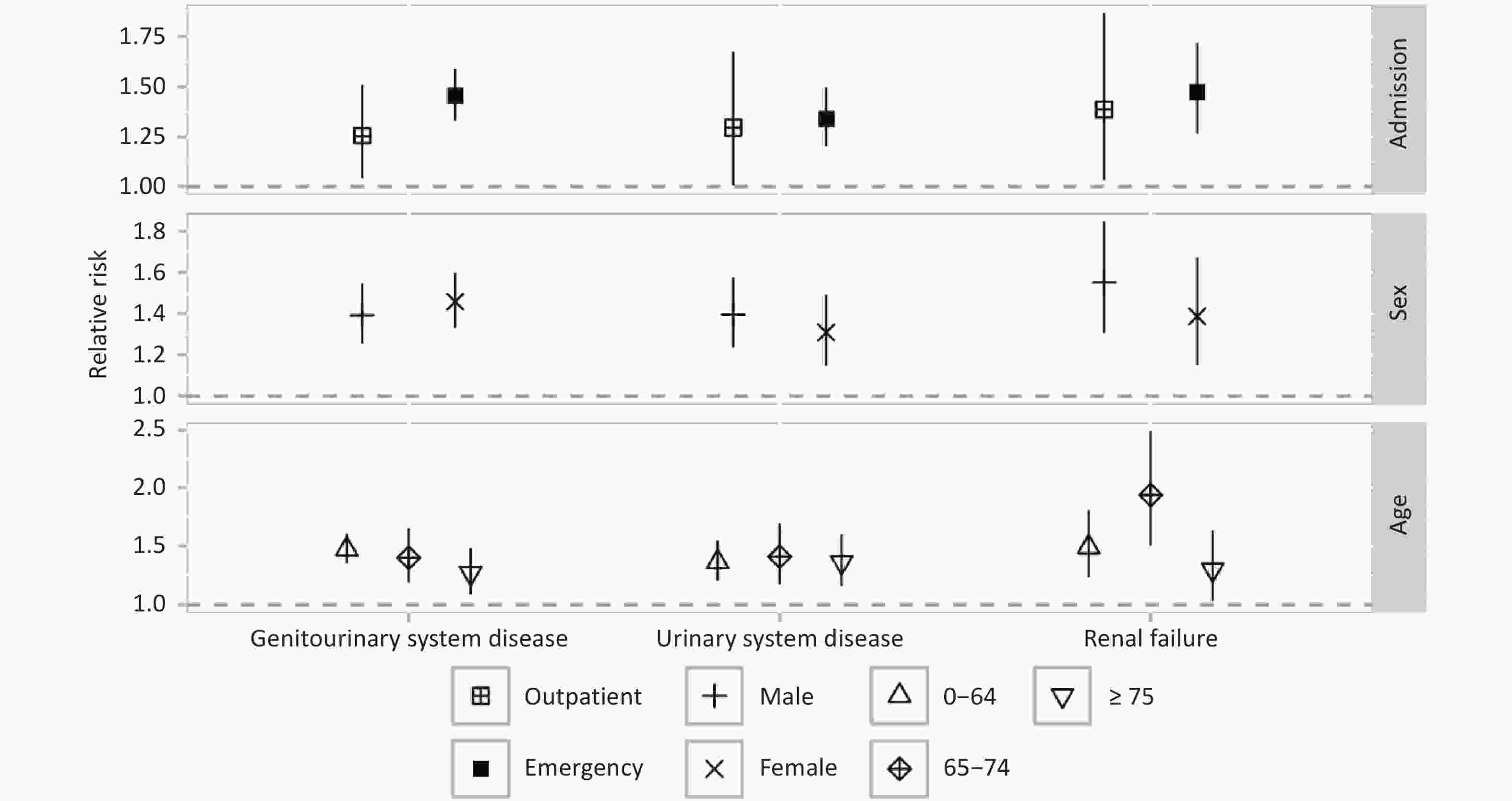
The emergency admission group had higher RR estimates for GUDs (RR = 1.45 (95% CI: 1.33–1.59)), urinary system disease (RR = 1.34 (95% CI: 1.20–1.50)), and renal failure (RR = 1.47 (95% CI: 1.27–1.72)) compared to the outpatient admission group (Figure 3) in p1day2 (lag10). Females exhibited a higher RR for GUDs hospital admissions (RR = 1.46 (95% CI: 1.33–1.60)) compared to males (RR = 1.39 (95% CI: 1.25–1.55)), while the RR estimates for urinary system disease and renal failure were lower in females, although these differences were not statistically significant. The RR estimate for GUDs hospital admission was highest in the 0-64 years age group (RR = 1.47 (95% CI: 1.35–1.61)) and lowest in the ≥ 75 years group (RR = 1.27 (95% CI: 1.08–1.48)). The highest RR for renal failure hospital admission was observed in the 65-74 years age group (RR = 1.94 (95% CI: 1.50–2.49)).
-
This multi-district time series study examined the relationship between cold spells and hospitalizations for GUDs. The findings reveal a notable increase in hospitalization risk for GUDs, urinary system diseases, and renal failure following short-term exposure to cold spells, with the highest risk observed on p1day2 cold spell days. Particularly concerning is the heightened risk of renal failure among individuals aged 65-74 years.
The study emphasizes that cold spells, particularly those extremely severe ones, can significantly increase hospitalization risk for GUDs. This is consistent with the trend of results observed in some previous studies. For instance, a study in Guangzhou, China, found that p5day2 cold spells significantly increased GUD-related mortality (overall RR for lags 0-27: 1.75, 95% CI: 1.14–2.68)[20]. A nationwide time-series analysis in Korea also noted associations between cold spells and hospital admissions and mortality from AKI[18]. However, these two prior studies differed from our study in health outcomes, exposures, and statistical methods, which makes direct comparisons of RR values challenging. Additionally, recent studies on low-temperature exposure have corroborated statistically significant associations between cold exposure and increased kidney disease risk[28,29]. These studies corroborate our findings, reinforcing the association between cold exposure and renal disease risks. Thus, cold spells emerge as a significant risk factor for GUDs, particularly during extreme events. Therefore, prioritizing preventive measures against extreme cold spells is critical to mitigate the attributable burden of GUDs.
Several physiological mechanisms have been proposed to explain the link between cold exposure and urogenital disorders. One key mechanism is the increased risk of dehydration in cold environments, which can delay normal hemodynamic responses, impair cardiovascular function, and lead to renal hypoperfusion—a critical factor in kidney injury[24,30-32]. Additionally, cold-induced vasoconstriction and ischemia also promote endothelin release, which is associated with intimal thickening and AKI[33]. Besides, cold exposure stimulates sympathetic nerve activity, leading to renal arteriolar spasm and reduced renal blood flow, and cold exposure exacerbate blood pressure fluctuations, damage glomerular endothelial cells, and accelerate glomerulosclerosis and proteinuria, ultimately contributing to the development of CKD[16,19]. Beyond renal effects, cold exposure also impacts the reproductive system. Research suggests that low temperatures can disrupt ovarian function by altering serum luteinizing hormone (LH) levels and increasing luteinizing hormone receptor (LHR) expression[34]. Moreover, cold exposure may induce ovarian insulin resistance, resulting in hormonal imbalances that affect reproduction[35-38]. These mechanisms highlight that cold exposure not only increases the risk of kidney disease but also adversely affects reproductive health. Thus, cold spells may induce genitourinary injuries via proposed mechanisms such as dehydration-induced renal hypoperfusion, cold-induced vasoconstriction and reproductive dysfunction pathways. Therefore, comprehensive preventive strategies, including thermal protection, hydration and monitoring of reproductive health, may mitigate the adverse effects of cold exposure on GUDs.
This study revealed that the health risks associated with cold spells vary depending on the intensity of the cold spell, both in terms of risk level and lag effects. The risk of hospitalization for GUDs increases with the intensity of the cold spell. Regarding lag effects, the risk of extreme cold spells (e.g., p1day2, p2day2) exhibited a parabolic pattern, rising initially, peaked at lag 10, and then declining over time. The effect persists for nearly two weeks. However, for milder cold spells (e.g., p3day2, p4day2, p5day2), the risk fluctuated, decreasing and then rising again. This fluctuation may be attributed to the occurrence of a more intense cold spell during the lag period of a milder one, complicating the interpretation of risk patterns. Consequently, we advocate for the implementation of interventions within a 13-day period following the onset of a cold spell, particularly in instances of an extreme cold spell, to impede the manifestation of early symptoms that have the potential to ultimately result in GUDs.
Our analysis further revealed that the risk of hospitalization for renal failure during cold spells was greater than the risk for general GUDs. This increased risk of hospitalization for renal failure was statistically significant starting from 7 days after cold spell onset and persisted until day 12. These findings suggest that proactive health protection measures at the onset of a cold spell or timely therapeutic interventions during the lag period could help mitigate the occurrence of renal failure. Renal failure is often linked to cardiovascular and kidney diseases, and numerous studies have reported an association between cold spells and elevated blood pressure, with the kidneys being one of the primary organs affected by hypertension[39]. This connection may help explain the heightened risk of renal failure observed in this study. The increase in hospitalizations for renal failure underscores the importance of monitoring and implementing interventions, for at least two weeks following extreme cold spell events.
This study observed that individuals aged 65-74 years are at a significantly elevated risk of hospitalization for renal failure during cold spells, consistent with Japanese studies reporting an elevated risk of mortality from cold-related kidney disease in individuals aged 65 years and older[28]. This heightened risk likely stems from a combination of increased vulnerability and greater exposure to cold weather. Older adults, particularly those in this age group, are more susceptible to cold-related health issues due to the natural decline in their ability to sense low temperatures and regulate body heat as they age[40,41]. Furthermore, individuals aged 65-74 often have higher rates of comorbidities, such as hypertension and diabetes, both of which are established risk factors for kidney disease[42]. In contrast to individuals aged 75 and older, those aged 65-74 may also engage in more outdoor activities, increasing their exposure to cold conditions and potentially raising their risk of cold-induced kidney damage. Given the global trend of population aging and the projected rise in the number of people aged 65 and older, the burden of cold spell-related renal failure is likely to increase. This highlights the need for proactive, early preventive strategies to mitigate the risk of renal failure during cold spells. Public health interventions should prioritize at-risk populations to reduce the incidence of cold-related kidney disease and subsequent hospitalizations.
This study has several limitations. Firstly, the time series analysis employed was an ecological research method, which inherently carries some limitations in terms of causal inference. Secondly, potential exposure misclassification may arise because cold spell exposure was measured at the city level rather than at the individual level. This approach is not equipped to take into account the heterogeneity of population characteristics across different districts, nor does it incorporate individual-level exposure character. Thirdly, the present study defined cold spells on the basis of percentile of daily mean temperature thresholds. However, it is important to note that this may not fully capture the health impacts of temperature decline intensity.
-
Our findings demonstrate that extreme cold spells significantly increase the risk of hospitalization for GUDs in general, urinary system diseases and renal failure, supporting the need for public health measures to address cold spell-related health risks. Additionally, the study identifies older adults aged 65–74 years as a vulnerable group, thereby laying a foundation for targeted research and interventions aimed at mitigating cold spell related health risks.
doi: 10.3967/bes2025.127
Risk of Hospitalization for Genitourinary System Diseases Following Exposure to Cold Spells
-
Abstract:
Objective To assess relationships between cold spells and genitourinary hospitalization risk. Methods Hospitalization records for genitourinary system diseases (GUDs) from 16 districts in Beijing (2013–2018) were analyzed. Cold spells were defined based on varying intensity thresholds. A two-stage analytical method was employed: first, generalized linear models assessed district-specific associations between cold spells and hospitalizations; second, random-effects meta-analysis aggregated the district-level results. Subgroup analyses were performed by admission type (emergency vs. outpatient), age, and sex. Results A total of 271,579 GUD-related hospitalizations were recorded. Cold spells (p1day2, daily mean temperature below the 1st percentiles of the daily mean temperature distribution from January 1, 2013, to December 31, 2018, lasting for two or more consecutive days) were linked to a significant rise in hospitalization risks: 1.35 (95% CI: 1.23–1.49) for all GUDs, 1.43 (95% CI: 1.32–1.56) for urinary system diseases, and 1.46 (95% CI: 1.28–1.67) for renal failure, when compared to non-cold spell days. Emergency admissions showed higher risk increases than outpatient admissions. Conclusion Extreme cold spells significantly elevate hospitalization risks for GUDs. This highlights the urgent need for targeted public health interventions to mitigate cold-related health impacts, especially for vulnerable populations. -
Key words:
- Cold spells /
- Genitourinary system diseases /
- Hospitalization risk /
- Subgroup analysis
The authors declare that they have no conflicts of interest in this work.
注释:1) Authors’ Contributions: 2) Competing Interests: -
Table 1. Daily district-level hospital admissions during the cold season (November to March) from 2013 to 2018 across the 16 districts of Beijing
Variables Mean ± SD Min P25 Median P75 Max Genitourinary system disease (N00-N99) 18.7 ± 19.7 0 5 11 26 168 Admission type Emergency admission 2.3 ± 2.5 0 0 2 3 23 Outpatient admission 16.4 ± 18.2 0 4 10 23 155 Sex Male 6.7 ± 6.9 0 2 4 9 59 Female 12 ± 13.4 0 3 7 17 109 Age 0–64 14.2 ± 15.2 0 4 9 20 124 65–74 2.1 ± 2.6 0 0 1 3 26 ≥ 75 2.4 ± 3.1 0 0 1 3 26 Urinary system disease (N00-N39) 7.6 ± 7.6 0 2 5 11 75 Admission type Emergency admission 1.4 ± 1.6 0 0 1 2 18 Outpatient admission 6.3 ± 6.6 0 2 4 9 66 Sex Male 4.3 ± 4.6 0 1 3 6 42 Female 3.3 ± 3.5 0 1 2 5 33 Age 0–64 4.5 ± 4.5 0 1 3 6 43 65–74 1.4 ± 1.7 0 0 1 2 18 ≥ 75 1.8 ± 2.4 0 0 1 2 18 Renal failure (N17-N19) 2.4 ± 2.7 0 0 1 4 19 Admission type Emergency admission 0.4 ± 0.8 0 0 0 1 7 Outpatient admission 2.0 ± 2.4 0 0 1 3 16 Sex Male 1.3 ± 1.7 0 0 1 2 12 Female 1.1 ± 1.5 0 0 1 2 12 Age 0–64 1.1 ± 1.4 0 0 1 2 11 65–74 0.5 ± 0.9 0 0 0 1 7 ≥ 75 0.8 ± 1.2 0 0 0 1 10 Note. SD, standard deviation. Table 2. Daily mean temperature during cold spell days in Beijing from 2013 to 2018
Definition Days/district, n Daily mean temperature, °C Mean ± SD Min P25 Median P75 Max p1day2 336 −8.6 ± 1.5 −14.3 −9.1 −8.1 −7.7 −7.2 p2day2 576 −7.6 ± 1.9 −14.3 −8.3 −7.5 −6.2 −5.4 p3day2 864 −6.8 ± 1.8 −14.3 −7.8 −6.1 −5.4 −4.7 p4day2 1184 −6.2 ± 1.9 −14.3 −7.4 −5.6 −4.8 −3.9 p5day2 1520 −5.7 ± 1.9 −14.3 −6.8 −5.2 −4.3 −3.3 Note. SD, standard deviation. -
[1] Francis A, Harhay MN, Ong ACM, et al. Chronic kidney disease and the global public health agenda: an international consensus. Nat Rev Nephrol, 2024; 20, 473−85. [2] Nat Rev Nephrol. Kidney disease: a global health priority. 2024; 20, 421-3. [3] Jager KJ, Kovesdy C, Langham R, et al. A single number for advocacy and communication—worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant, 2019; 34, 1803−5. doi: 10.1093/ndt/gfz174 [4] GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet, 2016; 388, 1459−544. doi: 10.1016/S0140-6736(16)31012-1 [5] GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet, 2020; 395, 709−33. doi: 10.1016/S0140-6736(20)30045-3 [6] Flores-Mireles AL, Walker JN, Caparon M, et al. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol, 2015; 13, 269−84. doi: 10.1038/nrmicro3432 [7] Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation, 2003; 108, 2154−69. doi: 10.1161/01.CIR.0000095676.90936.80 [8] Matsushita K, Coresh J, Sang YY, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol, 2015; 3, 514−25. doi: 10.1016/S2213-8587(15)00040-6 [9] Couser WG, Remuzzi G, Mendis S, et al. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int, 2011; 80, 1258−70. doi: 10.1038/ki.2011.368 [10] Ene-Iordache B, Perico N, Bikbov B, et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health, 2016; 4, e307−19. doi: 10.1016/S2214-109X(16)00071-1 [11] Soto-Aguilera CA, Meneses-León J, Villaverde P, et al. Association between advanced glycation end products and estimated glomerular filtration rate: a cross-sectional analysis. Diabetol Metab Syndr, 2025; 17, 165. doi: 10.1186/s13098-025-01741-5 [12] Oliveira CL, Fernandez-Llimos F, Alves da Costa F, et al. A case/non-case study of a national pharmacovigilance database to explore drug-induced acute kidney injury. Int J Clin Pharm, 2025. [13] Romanowski K, Clark EG, Levin A, et al. Tuberculosis and chronic kidney disease: an emerging global syndemic. Kidney Int, 2016; 90, 34−40. doi: 10.1016/j.kint.2016.01.034 [14] Burkart KG, Brauer M, Aravkin AY, et al. Estimating the cause-specific relative risks of non-optimal temperature on daily mortality: a two-part modelling approach applied to the Global Burden of Disease Study. Lancet, 2021; 398, 685−97. doi: 10.1016/S0140-6736(21)01700-1 [15] Mikkelsen TS, Toft P. Prognostic value, kinetics and effect of CVVHDF on serum of the myoglobin and creatine kinase in critically ill patients with rhabdomyolysis. Acta Anaesthesiol Scand, 2005; 49, 859−64. doi: 10.1111/j.1399-6576.2005.00577.x [16] Ko A, Oh S, Byon JY, et al. Surviving the cold: assessing long-term outcomes among Korean CKD patients exposed to low perceived temperature during winter. Environ Res, 2024; 261, 119636. doi: 10.1016/j.envres.2024.119636 [17] Zimmerman JL, Shen MC. Rhabdomyolysis. Chest, 2013; 144, 1058−65. doi: 10.1378/chest.12-2016 [18] Kim KN, Shin MK, Lim YH, et al. Associations of cold exposure with hospital admission and mortality due to acute kidney injury: a nationwide time-series study in Korea. Sci Total Environ, 2023; 863, 160960. doi: 10.1016/j.scitotenv.2022.160960 [19] Sellers AJ, van Beek SMM, Hashim D, et al. Cold acclimation with shivering improves metabolic health in adults with overweight or obesity. Nat Metab, 2024; 6, 2246−53. doi: 10.1038/s42255-024-01172-y [20] Chen JJ, Dong H, Yang J, et al. The impact of cold spells on mortality from a wide spectrum of diseases in Guangzhou, China. Environ Res Lett, 2021; 16, 015009. doi: 10.1088/1748-9326/abd26f [21] Beijing Municipal Bureau of Statistics. Statistical Communiques. Beijing Statistical Yearbook 2017. https://nj.tjj.beijing.gov.cn/nj/main/2017-tjnj/zk/e/indexch.htm. [2017-11-08] [22] United Nations Department of Economic and Social Affairs, Population Division. Publications. World Population Prospects 2024: Summary of Results. https://desapublications.un.org/publications/world-population-prospects-2024-summary-results. [2024-07-11] [23] Lei J, Chen RJ, Yin P, et al. Association between cold spells and mortality risk and burden: a nationwide study in China. Environ Health Perspect, 2022; 130, 027006. doi: 10.1289/EHP9284 [24] Kim E, Kim H, Kim YC, et al. Association between extreme temperature and kidney disease in South Korea, 2003-2013: stratified by sex and age groups. Sci Total Environ, 2018; 642, 800−8. doi: 10.1016/j.scitotenv.2018.06.055 [25] Liu ZR, Ma YX, Zhao YH, et al. Health effects of extreme low temperatures and cold waves on respiratory diseases. Biomed Environ Sci, 2024; 37, 682−5. [26] Liu C, Chen RJ, Sera F, et al. Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med, 2019; 381, 705−15. doi: 10.1056/NEJMoa1817364 [27] Dominici F, Peng RD, Bell ML, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA, 2006; 295, 1127−34. doi: 10.1001/jama.295.10.1127 [28] Htay ZW, Ng CFS, Kim Y, et al. Associations between short-term exposure to ambient temperature and renal disease mortality in Japan during 1979-2019: a time-stratified case-crossover analysis. Environ Epidemiol, 2024; 8, e293. doi: 10.1097/EE9.0000000000000293 [29] Talukder MR, Islam MT, Mathew S, et al. The effect of ambient temperatures on hospital admissions for kidney diseases in Central Australia. Environ Res, 2024; 259, 119502. doi: 10.1016/j.envres.2024.119502 [30] Kenefick RW, Hazzard MP, Mahood NV, et al. Thirst sensations and AVP responses at rest and during exercise-cold exposure. Med Sci Sports Exerc, 2004; 36, 1528−34. doi: 10.1249/01.MSS.0000139901.63911.75 [31] Lim YH, Park MS, Kim Y, et al. Effects of cold and hot temperature on dehydration: a mechanism of cardiovascular burden. Int J Biometeorol, 2015; 59, 1035−43. doi: 10.1007/s00484-014-0917-2 [32] Park MY, Ahn J, Bae S, et al. Effects of cold and hot temperatures on the renal function of people with chronic disease. J Occup Health, 2024; 66, uiae037. doi: 10.1093/joccuh/uiae037 [33] Yoshitomi Y, Kojima S, Ogi M, et al. Acute renal failure in accidental hypothermia of cold water immersion. Am J Kidney Dis, 1998; 31, 856−9. doi: 10.1016/S0272-6386(98)70057-5 [34] Xu T, Li X, Yang L, et al. Impact of cold exposure on the reproductive function in female rats. BioMed Res Int, 2018; 2018, 3674906. [35] Cankar K, Music M, Finderle Z. Cutaneous microvascular response during local cold exposure - the effect of female sex hormones and cold perception. Microvasc Res, 2016; 108, 34−40. doi: 10.1016/j.mvr.2016.07.006 [36] Stening K, Eriksson O, Wahren L, et al. Pain sensations to the cold pressor test in normally menstruating women: comparison with men and relation to menstrual phase and serum sex steroid levels. Am J Physiol Regul Integr Comp Physiol, 2007; 293, R1711−6. doi: 10.1152/ajpregu.00127.2007 [37] Dorfman M, Ramirez VD, Stener-Victorin E, et al. Chronic-intermittent cold stress in rats induces selective ovarian insulin resistance. Biol Reprod, 2009; 80, 264−71. doi: 10.1095/biolreprod.108.070904 [38] Zhang WY, Niu CJ, Jia H, et al. Effects of acute cold exposure on oxidative balance and total antioxidant capacity in juvenile Chinese soft-shelled turtle, Pelodiscus sinensis. Integr Zool, 2017; 12, 371−8. doi: 10.1111/1749-4877.12247 [39] Mulè G, Castiglia A, Cusumano C, et al. Subclinical kidney damage in hypertensive patients: a renal window opened on the cardiovascular system. Focus on microalbuminuria. Adv Exp Med Biol, 2017; 956, 279−306. [40] Young AJ, Lee DT. Aging and human cold tolerance. Exp Aging Res, 1997; 23, 45−67. doi: 10.1080/03610739708254026 [41] Smolander J. Effect of cold exposure on older humans. Int J Sports Med, 2002; 23, 86−92. doi: 10.1055/s-2002-20137 [42] Mallappallil M, Friedman EA, Delano BG, et al. Chronic kidney disease in the elderly: evaluation and management. Clin Pract, 2014; 11, 525−35. doi: 10.2217/cpr.14.46 -




 下载:
下载:



 Quick Links
Quick Links