-
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) belongs to the tumor necrosis factor (TNF) family and is considered as a promising anticancer agent due to its ability to selectively induce apoptosis in cancer cells while causing limited harm to normal tissue cells[1-2]. However, in a study conducted by Todaro and coworkers, approximately 50% of human cancer cell lines examined, including lung adenocarcinoma cells and a number of primary cancer cells were resistant to TRAIL, resulting in poor clinical therapeutic efficacy of TRAIL treatment[3]. Hence, an investigation of the molecular mechanisms of TRAIL resistance is necessary and urgent to improve the efficiency of TRAIL therapy.
The mitochondrial intrinsic apoptotic pathway and the death receptor-mediated extrinsic apoptotic signaling pathway are the main mechanisms to mediate apoptosis in the majority of cells. The death receptor signaling pathway is mainly activated in the process of TRAIL-induced apoptosis[4-5]. TRAIL causes apoptosis by binding to its death receptors, TRAIL-R1 (DR4) and TRAIL-R2 (DR5)[4-5] and recruiting the formation of death-inducing signaling complex (DISC)[6-7], which includes FADD (Fas-associated death domain protein) and procaspase 8 (or procaspase 10). Caspase 8 can be activated in the DISC complex, subsequently leading to the activation of downstream effector caspases, including caspases 3, which is the marker of apoptosis[7-8].
Some key factors such as protein kinase C (PKC)[9-12], NF-κB[9, 13-14], glycogen synthase kinase beta (GSK3β)[9, 15-18], cellular FLICE-inhibitory protein (c-FLIP)[12, 19-20], and X-linked IAP (XIAP)[21] have been demonstrated to be important for TRAIL-induced apoptosis. In our previous studies, we reported that PKCα phosphorylates and inactivates GSK3β, leading to the sensitization of H1299 non-small-cell lung cancer cells to TRAIL-induced apoptosis[9]. NF-κB mediates the anti-apoptotic effect of GSK3β[9]. Furthermore, we showed that hnRNPK (heterogeneous nuclear ribonucleoprotein K) could prevent the Ser9 phosphorylation of GSK3β by PKC, contributing to the resistance to TRAIL in H1299 cells[20]. The stability of c-FLIP protein mediates the anti-apoptotic effect of hnRNPK under conditions of TRAIL treatment[20].
hnRNPK is a conserved DNA and RNA binding ribonucleoprotein with the triple K-homology domain, which functions by regulating the transcription and post-transcriptional metabolism of RNA[22-23]. It has been reported that hnRNPK expression is augmented in a number of human cancers, including lung adenocarcinoma[24-28]. hnRNPK shuttles between the nucleus and cytoplasm[22-23, 29], and the abnormal cytoplasmic localization of hnRNPK has been frequently reported in human cancers. However, the function of cytoplasmic hnRNPK has not yet been fully elucidated.
Phosphorylation of hnRNPK in its K nuclear shuttling domain (KNS) plays an important role in nucleo-cytoplasmic shuttling by promoting the cytoplasmic localization of hnRNPK[13]. It has been reported that activated MAPK/ERK efficiently phosphorylates hnRNPK at Serines 284 and 353 and results in its cytoplasmic accumulation[14]. In the present study, we further confirm the importance of cytoplasmic hnRNPK in the resistance to TRAIL-induced apoptosis in H1299 cells and its regulation by ERK1/2 signaling.
Src kinase, which plays important roles in cancer, has also been shown to phosphorylate hnRNPK and regulate its activity. For example, Src interacts with hnRNPK and phosphorylates its multiple Tyrosine residues such as Tyr230, Tyr234, and Tyr236. Furthermore, the phosphorylation of hnRNPK by Src inhibits its binding with the target mRNA and modulates the translation of the latter[30-32]. All these studies depict important interactions or cross-talk between hnRNPK and multiple kinases, thereby regulating the determination of cell fate.
-
Lung adenocarcinoma H1299 cells (Institute of Life Science Chinese Academy of Sciences, Shanghai, China) were maintained in Dulbecco's Modified Eagle medium (Gibco BRL, Grand Island, NY) with 10% fetal bovine serum (PAN-Biotech, Germany), in a humidified atmosphere containing 5% CO2 at 37 ℃ as described previously[9, 20].
-
The sense strand sequences of siRNA used in this study are as follows as described previously[20]: hnRNPK siRNA1: 5'-UAUUAAGGCUCUCCGUACATT-3', hnRNPK siRNA2: 5'-CCUUAUGAUCCCAACUUUUTT-3', and control siRNA (NC): 5'-UUCUCCGAACGUG UCACGUTT-3'. hnRNPK siRNA1 and hnRNPK siRNA2 were combined for transfection of H1299 cells. After 48 h of transfection, the cells were harvested.
-
GFP-hnRNPK plasmid was produced by PCR from Flag-hnRNPK using the primers: 5'-CGCTCGAGATG GAAACTGAACAGCCAGAA-3' (forward), and 5'-CCGG AATTCGGAATCCTTCAACATCTGC-3' (reverse). GFP-hnRNPK S284/353A and GFP-hnRNPK S284/353D were produced with the Site-Directed Gene Mutagenesis Kit (Beyotime, China). H1299 cells at a confluence of 80%-90% were transfected with the corresponding plasmid as indicated using Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen, Carlsbad, CA).
-
After the appropriate treatments, H1299 cells were subjected to the extraction of nuclear and cytoplasmic proteins. Specifically, 250 μL extraction buffer (10 mmol/L Tris-HCl, 10 mmol/L KCl, 5 mmol/L MgCl2, pH 7.6) was added to the cells. Then, 0.6% Triton X-100 was added for 30 min to disrupt the cell membrane. A volume of 250 μL Nuclear Isolation Buffer (10 mmol/L Tris-HCl, 10 mmol/L KCl, 5 mmol/L MgCl2, 0.35 mol/L sucrose) was added, and density gradient centrifugation was performed for 10 min. The supernatant contained cytoplasmic components, and the precipitation contained nuclear components. The supernatant was transferred to another centrifuge tube, and four volumes of pre-chilled acetone were added at -20℃ and incubated overnight. Next, the supernatant was centrifuged at 4℃ and 12, 000 rpm for 30 min, and the precipitation was dissolved in SDS lysis buffer (Beyotime, China) at 4℃ and 12, 000 rpm for 30 min. After centrifugation, the supernatant was the nuclear protein solution. The protein concentration was determined by BCA assay.
-
H1299 cells in the exponential growth phase were seeded in 6-well plates at 50% confluence and cultivated overnight. TRAIL (Sino Biological Inc. Beijing, China) was added to the cells at 50 ng/mL to achieve 80% cytotoxicity. When the cells returned 50% confluence, they were treated with 60 ng/mL TRAIL. After several rounds of TRAIL administration, the dose was increased to 100 ng/mL. The cells resistant to 100 ng/mL TRAIL were defined as TRAIL-resistant H1299 cells. The whole process lasted for one month.
-
H1299 cells transfected with or without GFP-hnRNPK, GFP-hnRNPK S284/353A, and GFP-hnRNPK S284/353D plasmid were treated or not with TRAIL (20 ng/mL), or U0126 (10 μmol/L, Beyotime, China) as indicated. Immunofluorescence (IF) was performed as described previously[33]. The primary hnRNPK antibody (1:100 dilution) and the Alexa Fluor 488-conjugated secondary antibodies (ZSGB-BIO, China) were used for detection. DAPI staining was used to determine the morphology of cell nuclei.
Imaging experiments were performed on confocal laser scanning microscopes (LSM700, Zeiss, Jena, Germany) equipped with a Zeiss Plan-Neofluar 40×/1.3 NA Oil DIC objective as described previously[33-34]. The images are representative of at least three independent experiments.
-
H1299 cells were transfected with the corresponding plasmids or siRNA, combined with TRAIL (20 ng/mL) or U0126 (2.5-40 μmol/L) treatments as indicated. Total cell lysate was prepared with buffer containing 20 mmol/L Tris, pH 7.5, 150 mmol/L NaCl, 1% Triton X-100, sodium pyrophosphate, β-glycerophosphate, EDTA, Na3VO4, leupeptin, and 1% protease inhibitor cocktail (Roche). Proteins (20-50 μg) were separated by 10% or 12% SDS-PAGE.
Blots were probed with antibodies against hnRNPK (sc-28380, SANTA CRUZ), green fluorescent protein (GFP, Beyotime, China), cleaved caspase 3 (Cell Signaling Technology), phosphate dehydrogenase (GAPDH, ZSGB-BIO, China), ERK1/2 (Cell Signaling Technology), phosphorylated ERK1/2 (Cell Signaling Technology), lamin-A (Proteintech, China), and beta-tubulin (Proteintech, China) following the standard protocol[35]. Quantitative analysis of immunoblotting data was performed with Image J software. The images are representative of at least three independent experiments.
-
After transfection with GFP-hnRNPK S284/353D and treatment with TRAIL, total RNA of H1299 cells was extracted with TRIzol (Invitrogen) and reverse transcribed into cDNA using iScriptTM cDNA Synthesis Kit (Bio-Rad)[36]. The SsoFast EvaGreen Supermix (Bio-Rad) was used to perform qRT-PCR with qRT-PCR system (Mini Opticon, Bio-Rad). The following specific primers were used: XIAP, 5'-GGCGACACTTTCCTAATTGC-3' (forward) and 5'-CTGCCATGGATGGATTTCTT-3' (reverse); ACTB, 5'-ACGTGGACATCCGCAAAG-3' (forward) and 5'-GACTCGTCATACTCCTGCTTG-3' (reverse). β-actin was used as the internal control. All analyses are based on experiments performed in triplicate.
-
Statistical analysis using Student's t-test was performed, and statistical significance was defined as P < 0.05.
-
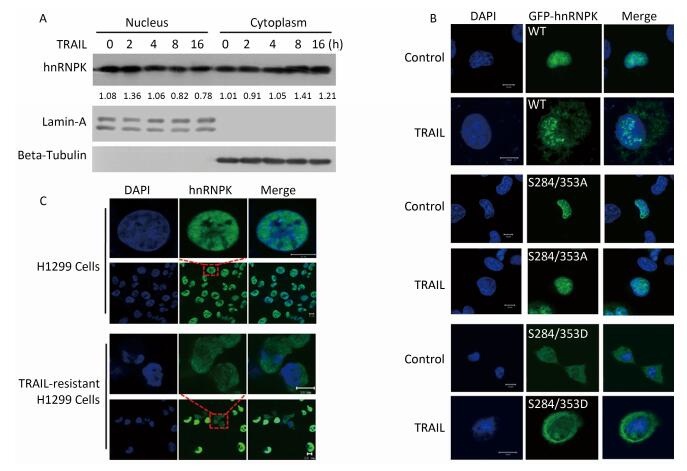
In our previous study[20], hnRNPK protein was shown to antagonize TRAIL-induced apoptosis. TRAIL treatment at 20 ng/mL for 8-12 h was sufficient to induce significant apoptosis in H1299 cells[20]. In this study, we also used a concentration of 20 ng/mL and 8 h of TRAIL stimulation. After TRAIL treatment, nuclear and cytoplasmic protein extraction was performed to identify the location of hnRNPK. As shown in Figure 1A, upon TRAIL stimulation, hnRNPK protein gradually accumulated in the cytoplasm in a month. As shown in Figure 1C, TRAIL-resistant H1299 cells displayed strong cytoplasmic expression of hnRNPK compared with normally cultured H1299 cells. Taken together, these results confirmed that TRAIL promoted cytoplasmic accumulation of hnRNPK by regulating the phosphorylation of S284 and S353 residues.
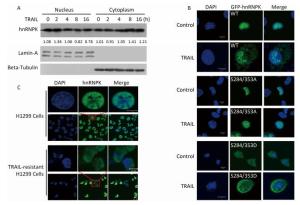
Figure 1. TRAIL promotes cytoplasmic accumulation of hnRNPK. (A) Nuclear and cytoplasmic protein extraction experiment was used to analyze the cellular localization of hnRNPK after TRAIL treatment. H1299 cells were treated with TRAIL as indicated and nuclear and cytoplasmic protein extraction performed as described in materials and methods. Western blotting was carried out to analyze the corresponding protein expression. (B) IF was used to analyze the cellular localization of exogenous hnRNPK. H1299 cells were transfected with GFP-hnRNPK, GFP-hnRNPK S284/353A, or GFP-hnRNPK S284/353D plasmid and treated with TRAIL (20 ng/mL, 8 h) then subjected to IF analysis. (C) The cellular localization of hnRNPK in TRAIL-resistant H1299 cells. The TRAIL-resistant H1299 cells were screened as described in materials and methods. IF assay was performed on H1299 cells and TRAIL-resistant H1299 cells with hnRNPK antibody and Alexa Fluor 488-conjugated secondary antibody. Bar: 10 μm.
-
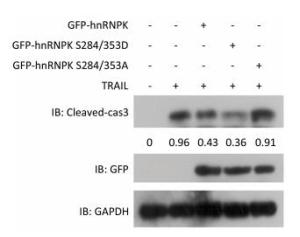
To further investigate the importance of subcellular localization of hnRNPK on apoptosis induced by TRAIL, H1299 cells were transfected with GFP-hnRNPK, GFP-hnRNPK S284/353A, or GFP-hnRNPK S284/353D plasmid and then treated with TRAIL for 8 h. The level of cleaved caspase 3 detected by Western blotting was used to assess the degree of cell apoptosis. The results in Figure 2 show that overexpression of wildtype GFP-hnRNPK decreased the cleaved caspase 3 level and partly prevented TRAIL-induced apoptosis. Interestingly, overexpression of GFP-hnRNPK S284/353D (cytoplasmic) caused a greater reduction of caspase 3 cleavage and largely antagonized TRAIL-induced apoptosis, whereas the GFP-hnRNPK S284/353A (nuclear) mutant had little effect on the cleavage of caspase 3 (Figure 2). These results demonstrated that the antagonizing effect of hnRNPK toward TRAIL-induced apoptosis is mediated mainly by its cytoplasmic localization.
-
It has been reported that ERK1/2 kinase could phosphorylate hnRNPK at S284 and S353 sites and promote cytoplasmic accumulation of hnRNPK under conditions of serum starvation in HeLa cells[14]. Here we tested whether ERK1/2 was involved in the cytoplasmic accumulation induced by TRAIL in H1299 cells. U0126, an inhibitor of ERK1/2, was used at a concentration of 10 μmol/L for this purpose to effectively inhibit the phosphorylation of ERK1/2 (Figure 3A). Figure 3B shows that U0126 significantly prevented the cytoplasmic localization of hnRNPK induced by TRAIL. A cell fractionation experiment was used to further confirm the inhibitory effect of U0126 pretreatment on the nucleo-cytoplasmic translocation of hnRNPK (Figure 3C). Taken together, these results show that ERK1/2 is involved in the cytoplasmic accumulation of hnRNPK after TRAIL stimulation.
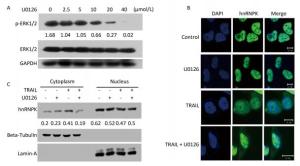
Figure 3. ERK1/2 inhibited cytoplasmic accumulation of hnRNPK induced by TRAIL. (A) The effects of U0126 on the activation of ERK1/2. H1299 cells were treated with U0126 with the indicated concentrations for 8 h. Then, the cells were harvested and subjected to Western blot analysis with the indicated antibodies. (B) U0126 prevented cytoplasmic accumulation of hnRNPK induced by TRAIL. H1299 cells were treated with U0126 (10 μmol/L) and/or TRAIL for 8 h, then subjected to IF assay with hnRNPK antibody and Alexa Fluor 488 conjugated secondary antibody. Bar: 10 μm. (C) Nuclear and cytoplasmic protein extraction experiment was used to analyze the cellular localization of hnRNPK after TRAIL combined with U0126 treatments. H1299 cells were treated with U0126 (10 μmol/L) and/or TRAIL for 8 h, then subjected to nuclear and cytoplasmic protein extraction. Western blotting was carried out to analyze the corresponding protein expression. The values represent the hnRNPK/beta-Tubulin or hnRNPK/lamin-A ratio of the gray values for the protein bands.
-
Next, we investigated whether ERK1/2-hnRNPK signaling was involved in the regulation of apoptosis induced by TRAIL. To this end, we combined knockdown or overexpression of hnRNPK with U0126 in H1299 cells and evaluated the degree of apoptosis reflected by the level of cleaved caspase 3. As shown in Figure 4A, upon treatment with TRAIL, the cleaved caspase 3 level was increased on which U0126 had a further stimulating effect, accompanied by the decreased phosphorylation of ERK1/2. Similarly, hnRNPK-siRNA transfection reduced the expression of hnRNPK and enhanced TRAIL-induced cleavage of caspase 3 (Figure 4A), whereas hnRNPK knockdown further augmented the cleavage of caspase 3 (Figure 4A). Furthermore, GFP-hnRNPK S284/353D overexpression could reduce the proapoptotic effect of U0126 (Figure 4B), suggesting that hnRNPK acted as a downstream effector of ERK1/2 to antagonize TRAIL-induced apoptosis.
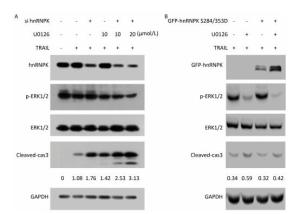
Figure 4. hnRNPK was a downstream effector of ERK1/2 in the antagonizing process of TRAIL-induced apoptosis. H1299 cells were transfected with hnRNPK siRNA (A) or GFP-hnRNPK S284/353D (B), and treated with U0126 and/or TRAIL for 8 h. Then, the cells were analyzed by Western blotting with the indicated antibodies.
-
It has been reported that hnRNPK could regulate the expression of XIAP, a key inhibitor of apoptosis[21]. We therefore tested if the subcellular translocation of hnRNPK during TRAIL-induced apoptosis might be involved in this regulation. To answer this question, H1299 cells were transfected with GFP-hnRNPK S284/353D alone or in combination with TRAIL treatment. The relative expression of XIAP was evaluated by RT-PCR. The results showed that TRAIL treatment reduced the expression of XIAP, while the overexpression of GFP-hnRNPK S284/353D significantly increased the expression of XIAP (Figure 5). Interestingly, overexpression of GFP-hnRNPK S284/353D reversed TRAIL-induced reduction of XIAP and caused a significant enhancement of XIAP expression. This result suggested that XIAP was a possible downstream effector of hnRNPK in the antagonizing process of TRAIL-induced apoptosis in H1299 cells.
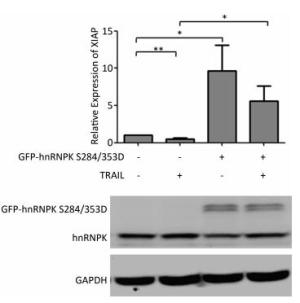
Figure 5. Cytoplasmic hnRNPK upregulates XIAP during TRAIL-induced apoptosis in H1299 cells. H1299 cells were transfected with GFP-hnRNPK S284/353D plasmid and treated with TRAIL for 8 h. The relative expression of XIAP mRNA was detected with qRT-PCR. The lower panel is the result of Western blotting assay of the corresponding samples. *P < 0.05, **P < 0.01.
-
TRAIL is a promising anticancer therapeutic agent, as it selectively causes cell death in cancer cells while producing negligible effects on normal cells[1-2]. However, resistance to TRAIL has turned out to be a general phenomenon, which greatly limits the efficiency of TRAIL therapy. Elucidating the molecular mechanisms of TRAIL action will provide new clues for sensitization of cancer cells to TRAIL-induced apoptosis[37]. TRAIL belongs to the TNF family. It induces cell apoptosis through binding to the death receptors, TRAIL-R1 (DR4) and TRAIL-R2 (DR5), which activates death receptor signaling pathways[4-5]. Upon binding with TRAIL, death receptors oligomerize and recruit the cytoplasmic proteins FADD and procaspase-8 (or procaspase-10) to form the DISC[6-7]. The auto-activation of caspase 8 in the complex leads to the subsequent activation of effector caspases, including caspases 3, 6, and 7, thereby inducing cell apoptosis[7-8]. The regulation of the TRAIL pathway involves multiple factors and has not yet been fully understood. XIAP has been shown to be a regulator of TRAIL sensitivity and represents a potential target of TRAIL combinatorial therapy[38-39].
An early study has reported that hnRNPK inhibited TRAIL-induced apoptosis through upregulation of c-FLIP transcription[40]. In our previous studies, we showed that hnRNPK antagonized TRAIL-induced apoptosis through inhibiting PKC-mediated GSK3β phosphorylation and c-FLIP stabilization[20]. In this study, we further investigated the involvement of cytoplasmic hnRNPK in the antagonism of TRAIL-induced apoptosis in H1299 cells, and confirmed that the ERK1/2-mediated phosphorylation of S284/353 in hnRNPK is important for this process. TRAIL promoted cytoplasmic accumulation of hnRNPK, which could be blocked by the ERK1/2 inhibitor U0126. Furthermore, we showed that the cytoplasmic hnRNPK increased the mRNA level of XIAP, suggesting that XIAP might be an effector of hnRNPK during TRAIL-induced apoptosis.
Cytoplasmic expression of hnRNPK was upregulated in many human cancers and involved in the antagonism of cancer cell apoptosis. Recent report demonstrated that cytoplasmic expression of hnRNPK significantly increased in human malignant melanoma[10]. Ionizing radiation induced the upregulation of cytoplasmic expression of hnRNPK. Pharmacological interference of ERK phosphorylation diminished cellular hnRNPK levels and sensitized melanoma cells to apoptosis stimulation[10]. Similarly, the cytoplasmic level of hnRNPK is correlated with the increased expression of thymidine phosphorylase (TP) in nasopharyngeal carcinoma (NPC)[11]. Moreover, the high levels of these two proteins predicted a poor prognosis in NPC. Interestingly, both MEK signaling inhibitor and the hnRNPK ERK-phosphoacceptor-site mutant diminished cytoplasmic expression of hnRNPK[11]. Furthermore, cytoplasmic expression of hnRNPK induced TP expression and allowed NPC cells to resist hypoxia-induced apoptosis[11]. In agreement with these findings, our previous study demonstrated that cytoplasmic levels of hnRNPK are significantly elevated in human lung adenocarcinoma[20]. In this study, our results showed that TRAIL treatment induced the upregulation of cytoplasmic levels of hnRNPK (Figure 1), which contributed H1299 cell resistance to TRAIL-induced apoptosis (Figure 2). Both the ERK1/2 signaling inhibitor U0126 and the ERK-phosphoacceptor-site mutant (GFP-hnRNPK S284/353A) diminished cytoplasmic expression of hnRNPK induced by TRAIL (Figure 3).
XIAP is a well-known potent caspase inhibitor[12]. It has been reported that hnRNPK antagonized TRAIL-induced apoptosis in HCC cells by maintaining a high expression level of XIAP[21]. hnRNPK knockdown reduced the expression of XIAP and enhanced TRAIL-induced caspase activity in HCC cells[21]. Recently, the enhanced expression of XIAP has been reported to mediate latent membrane protein 1 (LMP1)‑induced TRAIL resistance in NPC cells[39]. In our present study, we showed that overexpression of hnRNPK enhanced the expression of XIAP in H1299 cells, which possibly mediated hnRNPK‑induced TRAIL resistance (Figure 5).
In conclusion, we confirm the function and regulatory mechanism of cytoplasmic hnRNPK in TRAIL-induced apoptosis resistance, which gives new insight into the mechanisms of TRAIL resistance in lung adenocarcinoma.
-
HUANG Wen Si and XU Feng Mei carried out the majority of the experiments. ZENG Qing Zhong carried out the RT-PCR experiment. LIU Xiao Hui participated in the experiments regarding plasmid construction. GAO Xue Juan and LIU Lang Xia designed this work and drafted the manuscript.
doi: 10.3967/bes2017.063
ERK1/2-mediated Cytoplasmic Accumulation of hnRNPK Antagonizes TRAIL-induced Apoptosis through Upregulation of XIAP in H1299 Cells
-
Abstract:
Objective Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) resistance greatly limits the clinical therapeutic efficacy of TRAIL.Elucidating the molecular mechanism underlying TRAIL resistance will be fundamental to resolving this problem. Methods Nuclear and cytoplasmic protein extraction and immunofluorescence (IF) assay were used to detect changes in heterogeneous nuclear ribonucleoprotein K (hnRNPK) localization in H1299 cells.The evaluation of cell apoptosis in cells transfected with GFP-hnRNPK, GFP-hnRNPK S284/353A, or GFP-hnRNPK S284/353D mutant was performed using cleaved caspase-3 antibody.The gene expression of XIAP was tested by quantitative RT-PCR. Results Previously, we reported that hnRNPK antagonized TRAIL-induced apoptosis through inhibition of PKC-mediated GSK3β phosphorylation.In this study, we further demonstrate that TRAIL treatment induces cytoplasmic accumulation of hnRNPK in H1299 cells.The hnRNPK localized in the cytoplasm has a higher capacity to antagonize TRAIL-induced apoptosis.Both ERK1/2 signaling inhibitor U0126 and ERK-phosphoacceptor-site mutant (GFP-hnRNPK S284/353A) diminish cytoplasmic accumulation of hnRNPK induced by TRAIL.Moreover, we show that XIAP is involved in hnRNPK-mediated TRAIL resistance in H1299 cells. Conclusion Taken together, these results give new insights into the understanding of the molecular mechanism associated with TRAIL resistance in lung adenocarcinoma. -
Figure 1. TRAIL promotes cytoplasmic accumulation of hnRNPK. (A) Nuclear and cytoplasmic protein extraction experiment was used to analyze the cellular localization of hnRNPK after TRAIL treatment. H1299 cells were treated with TRAIL as indicated and nuclear and cytoplasmic protein extraction performed as described in materials and methods. Western blotting was carried out to analyze the corresponding protein expression. (B) IF was used to analyze the cellular localization of exogenous hnRNPK. H1299 cells were transfected with GFP-hnRNPK, GFP-hnRNPK S284/353A, or GFP-hnRNPK S284/353D plasmid and treated with TRAIL (20 ng/mL, 8 h) then subjected to IF analysis. (C) The cellular localization of hnRNPK in TRAIL-resistant H1299 cells. The TRAIL-resistant H1299 cells were screened as described in materials and methods. IF assay was performed on H1299 cells and TRAIL-resistant H1299 cells with hnRNPK antibody and Alexa Fluor 488-conjugated secondary antibody. Bar: 10 μm.
Figure 3. ERK1/2 inhibited cytoplasmic accumulation of hnRNPK induced by TRAIL. (A) The effects of U0126 on the activation of ERK1/2. H1299 cells were treated with U0126 with the indicated concentrations for 8 h. Then, the cells were harvested and subjected to Western blot analysis with the indicated antibodies. (B) U0126 prevented cytoplasmic accumulation of hnRNPK induced by TRAIL. H1299 cells were treated with U0126 (10 μmol/L) and/or TRAIL for 8 h, then subjected to IF assay with hnRNPK antibody and Alexa Fluor 488 conjugated secondary antibody. Bar: 10 μm. (C) Nuclear and cytoplasmic protein extraction experiment was used to analyze the cellular localization of hnRNPK after TRAIL combined with U0126 treatments. H1299 cells were treated with U0126 (10 μmol/L) and/or TRAIL for 8 h, then subjected to nuclear and cytoplasmic protein extraction. Western blotting was carried out to analyze the corresponding protein expression. The values represent the hnRNPK/beta-Tubulin or hnRNPK/lamin-A ratio of the gray values for the protein bands.
Figure 4. hnRNPK was a downstream effector of ERK1/2 in the antagonizing process of TRAIL-induced apoptosis. H1299 cells were transfected with hnRNPK siRNA (A) or GFP-hnRNPK S284/353D (B), and treated with U0126 and/or TRAIL for 8 h. Then, the cells were analyzed by Western blotting with the indicated antibodies.
Figure 5. Cytoplasmic hnRNPK upregulates XIAP during TRAIL-induced apoptosis in H1299 cells. H1299 cells were transfected with GFP-hnRNPK S284/353D plasmid and treated with TRAIL for 8 h. The relative expression of XIAP mRNA was detected with qRT-PCR. The lower panel is the result of Western blotting assay of the corresponding samples. *P < 0.05, **P < 0.01.
-
[1] Zhuang H, Jiang W, Zhang X, et al. Suppression of HSP70 expression sensitizes NSCLC cell lines to TRAIL-induced apoptosis by upregulating DR4 and DR5 and downregulating c-FLIP-L expressions. J Mol Med (Berl), 2013; 91, 219-35. doi: 10.1007/s00109-012-0947-3 [2] Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med, 1999; 5, 157-63. doi: 10.1038/5517 [3] Todaro M, Lombardo Y, Francipane MG, et al. Apoptosis resistance in epithelial tumors is mediated by tumor-cell-derived interleukin-4. Cell Death Differ, 2008; 15, 762-72. doi: 10.1038/sj.cdd.4402305 [4] Kischkel FC, Lawrence DA, Chuntharapai A, et al. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity, 2000; 12, 611-20. doi: 10.1016/S1074-7613(00)80212-5 [5] Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity, 1995; 3, 673-82. doi: 10.1016/1074-7613(95)90057-8 [6] Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ, 2003; 10, 26-35. doi: 10.1038/sj.cdd.4401186 [7] Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer, 2008; 8, 782-98. doi: 10.1038/nrc2465 [8] Kaufmann SH, Hengartner MO. Programmed cell death:alive and well in the new millennium. Trends Cell Biol, 2001; 11, 526-34. doi: 10.1016/S0962-8924(01)02173-0 [9] Gao X, Xu F, Zhang HT, et al. PKCalpha-GSK3beta-NF-kappaB signaling pathway and the possible involvement of TRIM21 in TRAIL-induced apoptosis. Biochem Cell Biol, 2016; 94, 256-64. doi: 10.1139/bcb-2016-0009 [10] Eder S, Lamkowski A, Priller M, et al. Radiosensitization and downregulation of heterogeneous nuclear ribonucleoprotein K (hnRNP K) upon inhibition of mitogen/extracellular signal-regulated kinase (MEK) in malignant melanoma cells. Oncotarget, 2015; 6, 17178-91. doi: 10.18632/oncotarget.v6i19 [11] Chen LC, Liu HP, Li HP, et al. Thymidine phosphorylase mRNA stability and protein levels are increased through ERK-mediated cytoplasmic accumulation of hnRNP K in nasopharyngeal carcinoma cells. Oncogene, 2009; 28, 1904-15. doi: 10.1038/onc.2009.55 [12] Shiozaki EN, Shi Y. Caspases, IAPs and Smac/DIABLO:mechanisms from structural biology. Trends Biochem Sci, 2004; 29, 486-94. doi: 10.1016/j.tibs.2004.07.003 [13] Michael WM, Eder PS, Dreyfuss G. The K nuclear shuttling domain:a novel signal for nuclear import and nuclear export in the hnRNP K protein. EMBO J, 1997; 16, 3587-98. doi: 10.1093/emboj/16.12.3587 [14] Habelhah H, Shah K, Huang L, et al. ERK phosphorylation drives cytoplasmic accumulation of hnRNP-K and inhibition of mRNA translation. Nat Cell Biol, 2001; 3, 325-30. doi: 10.1038/35060131 [15] Kotliarova S, Pastorino S, Kovell LC, et al. Glycogen synthase kinase-3 inhibition induces glioma cell death through c-MYC, nuclear factor-kappaB, and glucose regulation. Cancer Res, 2008; 68, 6643-51. doi: 10.1158/0008-5472.CAN-08-0850 [16] Beurel E, Blivet-Van Eggelpoël MJ, Kornprobst M, et al. Glycogen synthase kinase-3 inhibitors augment TRAIL-induced apoptotic death in human hepatoma cells. Biochem Pharmacol, 2009; 77, 54-65. doi: 10.1016/j.bcp.2008.09.026 [17] Liao X, Zhang L, Thrasher JB, et al. Glycogen synthase kinase-3beta suppression eliminates tumor necrosis factor-related apoptosis-inducing ligand resistance in prostate cancer. Mol Cancer Ther, 2003; 2, 1215-22. https://www.omicsonline.org/references/glycogen-synthase-kinase3beta-suppression-eliminates-tumor-necrosis-factorrelated-apoptosisinducing-ligand-resistance-in-prostate-cancer-595289.html [18] Song L, Zhou T, Jope RS. Lithium facilitates apoptotic signaling induced by activation of the Fas death domain-containing receptor. BMC Neurosci, 2004; 5, 20. doi: 10.1186/1471-2202-5-20 [19] Chen S, Cao W, Yue P, et al. Celecoxib promotes c-FLIP degradation through Akt-independent inhibition of GSK3. Cancer Res, 2011; 71, 6270-81. doi: 10.1158/0008-5472.CAN-11-0838 [20] Gao X, Feng J, He Y, et al. hnRNPK inhibits GSK3beta Ser9 phosphorylation, thereby stabilizing c-FLIP and contributes to TRAIL resistance in H1299 lung adenocarcinoma cells. Sci Rep, 2016; 6, 22999. doi: 10.1038/srep22999 [21] Xiao Z, Ko HL, Goh EH, et al. hnRNP K suppresses apoptosis independent of p53 status by maintaining high levels of endogenous caspase inhibitors. Carcinogenesis, 2013; 34, 1458-67. doi: 10.1093/carcin/bgt085 [22] Choi HS, Hwang CK, Song KY, et al. Poly(C)-binding proteins as transcriptional regulators of gene expression. Biochem Biophys Res Commun, 2009; 380, 431-6. doi: 10.1016/j.bbrc.2009.01.136 [23] Lee PT, Liao PC, Chang WC, et al. Epidermal growth factor increases the interaction between nucleolin and heterogeneous nuclear ribonucleoprotein K/poly(C) binding protein 1 complex to regulate the gastrin mRNA turnover. Mol Biol Cell, 2007; 18, 5004-13. doi: 10.1091/mbc.E07-04-0384 [24] Barboro P, Repaci E, Rubagotti A, et al. Heterogeneous nuclear ribonucleoprotein K:altered pattern of expression associated with diagnosis and prognosis of prostate cancer. Br J Cancer, 2009; 100, 1608-16. doi: 10.1038/sj.bjc.6605057 [25] Matta A, Tripathi SC, DeSouza LV, et al. Heterogeneous ribonucleoprotein K is a marker of oral leukoplakia and correlates with poor prognosis of squamous cell carcinoma. Int J Cancer, 2009; 125, 1398-406. doi: 10.1002/ijc.v125:6 [26] Chen LC, Hsueh C, Tsang NM, et al. Heterogeneous ribonucleoprotein k and thymidine phosphorylase are independent prognostic and therapeutic markers for nasopharyngeal carcinoma. Clin Cancer Res, 2008; 14, 3807-13. doi: 10.1158/1078-0432.CCR-08-0155 [27] Carpenter B, McKay M, Dundas SR, et al. Heterogeneous nuclear ribonucleoprotein K is over expressed, aberrantly localised and is associated with poor prognosis in colorectal cancer. Br J Cancer, 2006; 95, 921-7. doi: 10.1038/sj.bjc.6603349 [28] Chen X, Gu P, Xie R, et al. Heterogeneous nuclear ribonucleoprotein K is associated with poor prognosis and regulates proliferation and apoptosis in bladder cancer. J Cell Mol Med, 2016. https://www.researchgate.net/publication/310515586_Heterogeneous_nuclear_ribonucleoprotein_K_is_associated_with_poor_prognosis_and_regulates_proliferation_and_apoptosis_in_bladder_cancer [29] Dreyfuss G, Matunis MJ, Pinol-Roma S, et al. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem, 1993; 62, 289-321. doi: 10.1146/annurev.bi.62.070193.001445 [30] Ostareck-Lederer A, Ostareck DH, Cans C, et al. c-Src-mediated phosphorylation of hnRNP K drives translational activation of specifically silenced mRNAs. Mol Cell Biol, 2002; 22, 4535-43. doi: 10.1128/MCB.22.13.4535-4543.2002 [31] Adolph D, Flach N, Mueller K, et al. Deciphering the cross talk between hnRNP K and c-Src:the c-Src activation domain in hnRNP K is distinct from a second interaction site. Mol Cell Biol, 2007; 27, 1758-70. doi: 10.1128/MCB.02014-06 [32] Sen B, Johnson FM. Regulation of SRC family kinases in human cancers. J Signal Transduct, 2011; 2011, 865819. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3135246/ [33] Gao X, Dan S, Xie Y, et al. 14-3-3ζ reduces DNA damage by interacting with and stabilizing proliferating cell nuclear antigen. J Cell Biochem, 2015; 116, 158-69. doi: 10.1002/jcb.v116.1 [34] Gao X, Wang JY, Gao LM, et al. Identification and analysis of glycogen synthase kinase 3 beta1 interactome. Cell Biol Int, 2013; 37, 768-79. doi: 10.1002/cbin.v37.8 [35] Gao X, He Y, Gao LM, et al. Ser9-phosphorylated GSK3β induced by 14-3-3ζ actively antagonizes cell apoptosis in a NF-κB dependent manner. Biochem Cell Biol, 2014; 92, 349-56. doi: 10.1139/bcb-2014-0065 [36] Gao X, He Y, Gao LM, et al. Ser9-phosphorylated GSK3beta induced by 14-3-3zeta actively antagonizes cell apoptosis in a NF-kappaB dependent manner. Biochem Cell Biol, 2014; 92, 349-56. doi: 10.1139/bcb-2014-0065 [37] Felber M, Sonnemann J, Beck JF. Inhibition of novel protein kinase C-epsilon augments TRAIL-induced cell death in A549 lung cancer cells. Pathol Oncol Res, 2007; 13, 295-301. doi: 10.1007/BF02940308 [38] Vogler M, Dürr K, Jovanovic M, et al. Regulation of TRAIL-induced apoptosis by XIAP in pancreatic carcinoma cells. Oncogene, 2007; 26, 248-57. doi: 10.1038/sj.onc.1209776 [39] Yang S, Li SS, Yang XM, et al. Embelin prevents LMP1-induced TRAIL resistance via inhibition of XIAP in nasopharyngeal carcinoma cells. Oncol Lett, 2016; 11, 4167-76. https://www.researchgate.net/publication/301944814_Embelin_prevents_LMP1-induced_TRAIL_resistance_via_inhibition_of_XIAP_in_nasopharyngeal_carcinoma_cells [40] Chen LC, Chung IC, Hsueh C, et al. The antiapoptotic protein, FLIP, is regulated by heterogeneous nuclear ribonucleoprotein K and correlates with poor overall survival of nasopharyngeal carcinoma patients. Cell Death Differ, 2010; 17, 1463-73. doi: 10.1038/cdd.2010.24 -




 下载:
下载:



 Quick Links
Quick Links