-
Microwave radiation, non-ionizing electromagnetic radiation (EMR) at frequencies ranging from 300 MHz to 300 GHz, has been widely used in industrial synthesis, medicine, communication, etc. In the past decades, microwave-induced injury has been demonstrated in several tissues and organs in animal models. Therefore, concerns over microwave-induced health hazards have been spreading[1-4]. Our group is interested in evaluating the biological effects of microwave exposure and investigating the mechanisms of microwave-induced injury of the central nervous and cardiovascular systems[5-11]. The immune system plays pivotal roles in the development and progression of various diseases. However, the effects of microwaves on the immune system are still a matter of debate. Several groups showed that microwaves could enhance the immune response, such as by increasing phagocytic activity of macrophages and promoting proliferation of T lymphocytes, while others presented opposite results due to the different frequency, dose, and exposure time[12-17].
Natural killer cells (NK), the major effectors of the innate immune system, also participate in activation of adaptive immune responses and induce antigen-specific immune responses[18]. It has been reported that NK cells decrease in men occupationally exposed to microwave expsoure at frequencies of 6-12 GHz[19]. However, functional injury of NK cells and its mechanisms are still unclear. NK cell-mediated cytotoxicity is the central event in the defense against pathogens and transformed cells. Importantly, NK cell-mediated cytotoxicity acts through the expression and release of perforin and granzyme B. Moreover, natural killer group 2D (NKG2D) receptor is a pivotal activating receptor on NK cells to activate cytotoxicity[20-22].
In this study, we will evaluate the effect of 10, 30, and 50 mW/cm2 microwaves on cellular apoptosis, the cell cycle, cytotoxicity of NK-92 cells in vitro, and investigate the roles of NKG2D and ERK signaling in microwave-induced injury of NK-92 cells.
-
The human NK cell line, NK-92, was kindly provided by Dr. Zhao Lian (Beijing Institute of Transfusion Medicine) and cultured in α-MEM media (Thermo Scientific, Wilmington, DE) supplemented with 12.5% fetal bovine serum (FBS) and 12.5% horse serum (Gibco, Grand Island, NY), plus 2000 U/mL interleukin-2 (Beijing Sihuan Pharmaceutical Co., Ltd, Beijing, China).
Human chronic myelogenous leukemia cell line, K562, which has been widely used as target cells for detecting cytotoxicity of human NK cells, was kindly provided by Dr. Li Changyan (Beijing Institute of Radiation Medicine) and were maintained in RPMI-1640 media (Thermo Scientific) plus 10% FBS.
-
NK-92 cells were exposed to microwaves at an average power density of 10, 30, and 50 mW/cm2 for 5 min. The microwave source was a klystron amplifier model JD 2000 (Vacuum Electronics Research Institute, Beijing, China), and microwave energy was transmitted by a rectangular wave guide and A16-dB standard-gain horn antenna to an electromagnetic shield chamber[23]. The NK92 cells were seeded in a 6-well plate and cultured for 24 h. Then, cells were placed on an appointed exposure table and exposed to microwaves with an average power density of 10, 30, and 50 mW/cm2 for 5 min. The sham group (SH group) was treated in the same place for 5 min, but without microwave exposure. All exposures were performed at room temperature in a shielded room.
-
NK-92 cells were exposed to 10, 30, and 50 mW/cm2 microwaves, and the morphological changes were observed on an inverted phase contrast microscope (Olympus Co., Tokyo, Japan). Moreover, the ultrastructure was observed by transmission electron microscopy (TEM). Briefly, cell samples (1 mm3 cubes) were collected at 1 h and 24 h after microwave exposure and then fixed in 2.5% glutaraldehyde, sequentially processed with 1% osmium tetroxide and a graded series of ethyl alcohols, and embedded in EPON618. Samples were cut into ultrathin (70-nm) sections for TEM. Ultrathin sections cut onto copper mesh grids were stained with heavy metals, uranyl acetate, and lead citrate for contrast. After drying, the grids were observed by using TEM (HITACHI Ltd, Tokyo, Japan).
-
Exponentially growing NK-92 cells were seeded at a density of 1 × 106 cells·mL-1·well-1 in 6-well plates and exposed to microwaves at a density of 0, 10, 30, and 50 mW/cm2. At 1 h and 24 h after exposure, total cells were harvested and analyzed with the Annexin V-FITC Apoptosis Detection Kit (SunGene Biotech, Tianjin, China) according to the manufacturer's instructions. Normal cultured NK-92 cells were used as a negative control. To confirm the mechanisms of microwave-induced apoptosis, the activity of NK cells was detected by using the Caspase-3 Activity Kit (Beyotime Institute of Biotechnology, Haimen, China) according to the manufacturer's protocol. Briefly, cell lysates were prepared at 0.5 h, 1 h, 6 h, and 12 h after microwave exposure. Lysates were incubated with caspase-3 substrate (Ac-DEVD-pNA) (2 mmol/L) at 37 ℃ for 12 h. Samples were measured at an absorbance of 405 nm. The detailed analysis procedure is described in the manufacturer's protocol.
-
Cytotoxicity of NK-92 Cells Exponentially growing NK-92 cells were exposed to 10, 30, or 50 mW/cm2 microwaves. One hour later, irradiated NK-92 cells were co-cultured with target cells (K562), at a ratio of 10:1 for 4 h. Then, lactate dehydrogenase (LDH) in the culture medium was detected by using an LDH assay kit (Jiancheng Bioengineering, Nanjing, China) according to the manufacturer's instructions.
Perforin and NKG2D Expression in NK-92 Cells Exponentially growing NK-92 cells were seeded at a density of 1 × 106 cells·mL-1·well-1 in 6-well plates. Twenty-four hours later, cells were exposed to 30 mW/cm2 microwaves. Then, cells were collected at 1 h, 6 h, and 12 h after exposure. The expression of perforin and NKG2D were detected by quantitative reverse transcription polymerase chain reaction (RT-PCR) and Western-blotting.
-
Exponentially growing NK-92 cells were exposed to 30mW/cm2 microwaves, cells were collected, and protein was extracted at 1 h, 6 h, and 12 h after exposure. The expression of phosphorylated ERK (p-ERK) was detected by Western-blotting and normalized to ERK expression.
For ERK signaling blockade experiments, the ERK-specific inhibitor U0126 (Cell Signaling Technology, Danvers, MA) was added to cultured NK-92 cells 30 min pre-irradiation, at a working concentration of 50 μmol/L, while DMSO was added to the control groups. Cells were collected at 1 h, 6 h, and 12 h after exposure, and the protein expression of perforin was measured by Western-blotting. Moreover, cells were collected at 1 h, 6 h, 12 h, and 24 h after exposure to analyze cellular apoptosis and the cell cycle as described above.
-
Total RNA was isolated from NK-92 cells with or without exposure to microwaves, and then complementary DNA (cDNA) was synthesized by using the AMV First Strand cDNA Synthesis Kit (Sangon Biotech, Shanghai, China). The expression of perforin and NKG2D were detected by using SYBR Green Real Master Mix (TIANGEN, Beijing, China) on a 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA) and normalized to human glycolytic glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primers used were as follows: GAPDH sense 5'-gaaggtgaaggtcggagtca-3' and anti-sense 5'-tggactccacgacgtactca-3'; NKG2D sense 5'-agccaggcttcttgtatgtctc-3' and anti-sense 5'-catccaatgatatgacttcacc-3'; perforin sense 5'-ctggcagggacgatgacct-3' and anti-sense 5'-gggaaccagacttgggagc-3'.
-
Protein was extracted from NK-92 cells with various treatments. After electroblotting onto a polyvinylidenedifluoride membrane (Millipore, Billerica, MA), the protein was labeled by rabbit anti-phospho-ERK1/2 antibody, rabbit anti-ERK1/2 antibody, mouse anti-human NKG2D antibody (Santa Cruz, Santa Cruz, CA), and rabbit anti-GAPDH antibody (KangChen Biology, Nanjing, China). Then, the membranes were incubated with corresponding horse radish peroxidase (HRP) conjugated secondary antibodies (Santa Cruz) and scanned by the MultiimageII system (Alpha Innotech, Silicon Valley, CA). The protein expression was normalized to that of GAPDH.
-
All data were based on duplicates of at least three independent experiments and analyzed with GraphPad Prism software version 5 (GraphPad software, San Diego, CA). All values are shown as mean ± SEM. Longitudinal data were analyzed by using a two-way repeated measure ANOVA followed by a Bonferroni post hoc test. Data including more than two groups were analyzed by one-way ANOVA followed by a Bonferroni post hoc test. A value of P < 0.05 was considered to denote statistical significance.
-
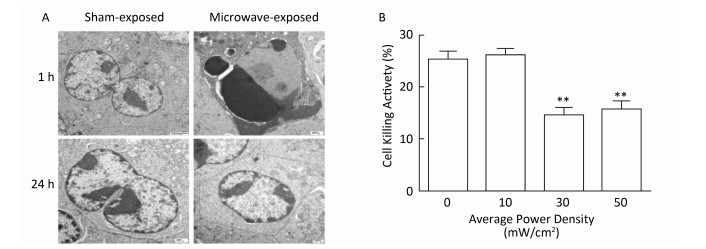
NK-92 cells were exposed to 10, 30, or 50 mW/cm2 microwaves, and a vague outline and low refractive index could be observed at 1 h after exposure and then rapidly recovered at 24 h after exposure (data not shown). Furthermore, pyknotic or karyorrhectic nucleiand apoptotic bodies were detected by using TEM at 1 h after 30 mW/cm2 microwave exposure. However, no significant changes could be detected at 24 h after exposure (Figure 1A).
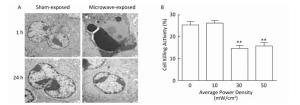
Figure 1. Microwave-induced morphological and functional injury of NK-92 cells. (A) NK-92 cells were exposed to 30 mW/cm2 microwaves, the ultrastructure of NK-92 cells was also observed at 1 h and 24 h after exposure by transmission electron microscopy (TEM). (B) NK-92 cells were exposed to 0 (Sham), 10, 30, and 50 mW/cm2 microwaves. One hour after exposure, NK-92 cells were collected and co-cultured with K562 cells at ratio of 10:1 for 4 h. Then, the lactate dehydrogenase (LDH) level in media was analyzed and the percentage of dead cells was calculated. **, P < 0.01 vs. Sham group.
NK cell-mediated cytotoxicity plays central and pivotal roles in immune defense against pathogens and transformed cells. Therefore, the cytotoxicity of NK-92 cells was analyzed by LDH assay after microwave exposure. Our data showed that 30 and 50 mW/cm2 microwaves, but not 10 mW/cm2 microwaves, decreased the cytotoxicity of NK-92 cells at 1 h after exposure (Figure 1B). These results suggested that 30 mW/cm2 and 50 mW/cm2 microwaves could induce both morphological and functional injury of NK cells.
-
Then, we analyzed the biological activities of NK-92 cells after microwave exposure. Our data showed that microwaves dose-dependently induced cellular apoptosis at 1 h after exposure. However, at 24 h after exposure, significant cellular apoptosis could only be induced by 50 mW/cm2 microwaves (Figure 2A-B). Consistent with cellular apoptosis, 30 mW/cm2 microwaves increased the activity of caspase 3 at 1 h after exposure and then returned it to normal levels (Figure 2C). However, no obvious changes in cellular proliferation could be detected (data not shown).
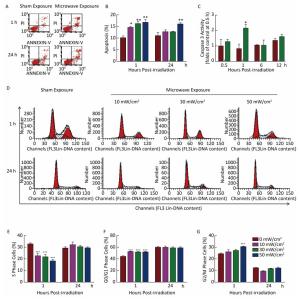
Figure 2. Microwave-induced cellular apoptosis and cell cycle arrest. NK-92 cells were exposed to 0 (Sham), 10, 30, and 50 mW/cm2 microwaves. At 1 h and 24 h after exposure, cells were collected and labeled with Annexin-V-FITC and PI. Then, cellular apoptosis was analyzed by flow cytometry. The representative images after 30 mW/cm2 microwave exposure are shown in (A). And the quantification of apoptosis rates is presented in (B). To confirm the mechanisms of microwave-induced apoptosis, the activity of NK cells was detected at 0.5 h, 1 h, 6 h, and 12 h after microwave exposure by using the Caspase-3 activity kit according to the manufacturer's protocol (C). For analysis of the cell cycle, cells were collected at 1 and 24 h after exposure, and fixed in 70% ethanol at -20 ℃ for 24 h. Then, cells were processed for cell cycle analysis by using flow cytometry. The representative images after 30 mW/cm2 microwave exposure are shown in (D). The percentages of cells in G0/G1 phase, in G2/M phase, and in S phase are shown in (E), (F), and (G), respectively. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. sham group at the same time point after exposure.
We also found that microwaves caused cell cycle arrest (Figure 2D-G). Briefly, microwave exposure increased the proportion of G0/G1 phase cells and decreased the proportion of S phase cells at 1 h after exposure, indicating that microwaves arrested cells in the G0/G1 phase. Moreover, 50 mW/cm2 microwave exposure also induced cell cycle arrest in G2/M phase. However, cell cycle arrest was abolished at 24 h post-exposure.
In short, 10, 30, and 50 mW/cm2 microwaves could induce cellular apoptosis and cell cycle arrest at 1 h, and then they rapidly returned to normal levels at 24 h after exposure.
-
NK cells mediated cytotoxicity and killing activities through perforin expression and release. In this study, we found that 30 mW/cm2 microwaves reduced perforin expression both at 1 h and 6 h after exposure, and then they were restored to normal levels (Figure 3A-B). At the mRNA level, microwaves also reduced perforin expression at 1 h after exposure (Figure 3D).
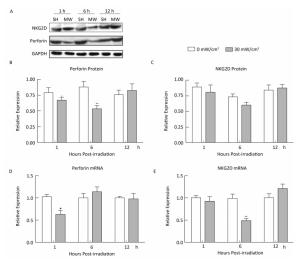
Figure 3. Microwaves regulate the expression of perforin and NKG2D in NK-92 cells. NK-92 cells were exposed to 0 (Sham) and 30 mW/cm2 microwaves. Protein was extracted from cells collected at 1 h, 6 h, and 12 h after exposure. The protein expression of perforin and NKG2D was analyzed by western-blotting (A) and the corresponding results of quantification are shown in (B) and (C). *, P < 0.05, **, P < 0.01 vs. sham group at the same time points after exposure. Cells were collected at 1 h, 6 h, and 12 h after exposure, and total RNA was isolated by using TRIzol reagent. After cDNA was synthesized, the mRNA expression of perforin (D) and NKG2D (E), an activating receptor on NK cells, were measured by quantitative reverse transcription polymerase chain reaction (RT-PCR).
NKG2D is an activating receptor on NK cells and is always upregulated on activated NK cells. In our previous studies, we found that NKG2D was downregulated in the spleen of Wistar rats after 30 mW/cm2 microwave exposure (data not shown). Although the mRNA expression of NKG2D decreased in NK-92 cells at 6h after exposure, no significant difference could be detected in protein expression (Figure 3C, 3E). These data indicated that microwaves could slightly downregulate NKG2D expression in NK cells.
-
Our previous reports suggested that ERK1/2 signaling plays pivotal roles in microwave-induced injury of various tissues and cells. To clarify the mechanisms of microwave-induced injury of NK-92 cells, ERK signaling was detected after 30 mW/cm2 microwave exposure. Microwaves significantly downregulated phosphorylated ERK (p-ERK) expression at 1 h after exposure, and then it was rapidly restored to normal levels (Figure 4A). Both microwaves and ERK signaling blockade could decrease perforin expression in NK-92 cells. Moreover, combining ERK blockade with microwave exposure inhibited perforin expression much more strongly, indicating that microwaves might regulate the cytotoxicity of NK-92 cells through ERK signaling (Figure 4B-C).
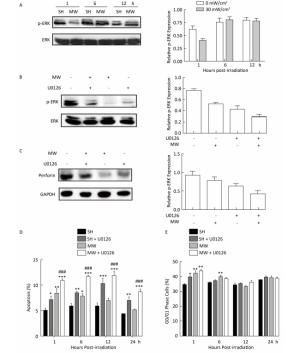
Figure 4. ERK signaling participates in microwave-induced injury of NK-92 cells. NK-92 cells were exposed to 0 (Sham) and 30 mW/cm2 microwaves. At 1 h, 6 h, and 12 h after exposure, cells were collected, and the expression of phosphorylated ERK (p-ERK) was detected by western-blotting. Then, p-ERK expression was normalized to total ERK (A). *, P < 0.05 vs. sham group at 1 h after exposure. To blocking ERK signaling, U0126 (a specific ERK inhibitor) was added to NK-92 cells 30 min before exposure at a concentration of 50 μmol/L. One hour after exposure to 0 (Sham) and 30 mW/cm2 microwaves, the level of p-ERK was confirmed by western-blotting and normalized to ERK (B), and the expression of perforin was also measured by western-blotting and normalized to GAPDH (C). The quantitative results of p-ERK and perforin are also shown. *, P < 0.05, **, P < 0.01 vs. sham group; ##, P < 0.01 vs. microwave exposure group (MW). Thirty minutes before microwave exposure, 50 μmol/L U0126 were added to NK-92 cells, using DMSO as a negative control. At 1 h, 6 h, 12 h, and 24 h after 0 and 30 mW/cm2 microwave exposure, cells were collected and used to analyze the cellular apoptosis and cell cycle by using flow cytometry. The apoptosis rates are shown in (D), and the percentage of cells in G0/G1 phase was calculated and displayed in (E). *, P < 0.05, **, P < 0.01, ***, P < 0.001 vs. sham group at the same time point; ##, P < 0.01, ###, P < 0.001 vs. microwave exposure group (MW) at the same time point.
Furthermore, we also analyzed whether ERK signaling was involved in microwave-induced cellular apoptosis and cell cycle arrest. Our data showed that both microwaves and U0126 could induce cellular apoptosis at 1 h after 30 mW/cm2 microwave exposure. However, at 24 h after exposure, significant apoptosis only could be detected in U0126-treated cells. Importantly, combined treatment could induce the strongest cellular apoptosis (Figure 4D). Although both U0126 and microwaves induced cell cycle arrest in G0/G1 phase, ERK blockade could not further promote microwave-induced G0/G1 phase arrest (Figure 4E). In short, our data suggest that ERK signaling participates in microwave-mediated apoptosis of NK-92 cells.
-
In this study, we showed that 30 mW/cm2 and 50 mW/cm2 microwaves induced cellular apoptosis and cell cycle arrest at 1 h after exposure, and significant cellular apoptosis could still be detected at 24 h after 50 mW/cm2 microwave exposure. These results suggested that long-term exposure to high-dose microwaves could induce injury of NK cells, which might lead to immune suppression and development of diseases, including infection and tumors. The immune system is one of the sensitive organs to microwaves, and microwave-induced apoptosis of lymphocytes, including T lymphocytes and B lymphocytes, has been demonstrated in vivo[24-25]. NK cells, the major effectors of the innate immune system, play pivotal roles in immune surveillance and defense against pathogens and tumors[26]. It has been reported that microwave exposure could reduce the number of NK cells in peripheral blood and immune organs, such as the thymus and spleen[27-28]. However, the mechanisms of NK cell decline were still unclear. Our data indicate that microwaves might reduce NK cells via induction of cellular apoptosis and death.
In this study, we also showed that 30 and 50 mW/cm2, but not 10 mW/cm2 microwave exposure, impaired the cytotoxicity of NK cells. NK cell-mediated cytotoxicity emerged as an activating marker of NK cells and plays pivotal roles in eliminating viral-infected cells and transformed tumor cells. The effect of microwaves on NK cell-mediated cytotoxicity remains controversial, and the results depend on the animal species, parameters of radiation, etc. In general, high-dose microwaves and/or long-time exposure decreased the cytotoxicity of NK cells, in agreement with the results of our study[17, 19, 28-29]. In NK cell-mediated cytotoxicity, the expression and release of perforin and granzymes are the central events. Briefly, after NK cell activation, perforin is released by NK cells to form pores on target cells, and then granzymesenter target cells to induce cell apoptosis and death. Our data showed that 30 mW/cm2 microwaves decreased perforin expression at 1 h and 6 h after exposure, which was consistent with cytotoxic activity. Moreover, NKG2D, the activating receptor of NK cells, can bind to ligands and activate downstream cell signaling pathways to elevate intercellular Ca2+, express and release perforin and granzymes, and finally, enhance NK cell-mediated cytotoxicity[30-31]. However, no significant changes in NKG2D protein expression were observed after 30 mW/cm2 microwave exposure in this study. Therefore, our results suggest that other mechanisms not mediated by NKG2D are involved in microwave-mediated impairment of cytotoxic activities.
Furthermore, in this study, we found that 30 mW/cm2 microwaves reduced the level of phosphorylated ERK in NK-92 cells. Moreover, ERK pathway blockade by U0126 led to much less perforin release and much greater cellular apoptosis after exposure to microwaves. ERK signaling is one of the important pathways to activate NK cell-mediated cytotoxicity[32-34]. Importantly, ERK signaling also participated in microwave-induced cell injury, as we have demonstrated previously in the central nervous system, both in vivo and in vitro[5, 10-11]. These data suggest that the ERK signaling pathway participates in microwave-induced injury of NK-92 cells via regulation of apoptosis and perforin expression.
Microwave radiation has emerged as the fastest growing environmental influence factors. Furthermore, anxiety and speculation about the potential health hazards of microwave exposure are growing and spreading. The immune system is one of the organs sensitive to microwaves[24-25]. Our findings suggest that microwave exposure could dose-and time-dependently induce NK cell injury, which might cause immune suppression. Therefore, microwave applications in telecommunications and medicine might induce immune suppression, which in turn may evoke or promote immune-related diseases. For example, using microwave ablation to treat tumors might induce immune suppression in the tumor microenvironment. To avoid immune suppression, the proper power density of microwaves must be identified. Moreover, combination with immunotherapy is also a possible strategy to improve the therapeutic effects[35-36].
-
In this study, 10, 30, and 50 mW/cm2 microwave exposure dose-dependently caused injury to NK-92 cells, including ultrastructural injury, cellular apoptosis, cell cycle arrest and decreased cytotoxic activity. As the major effectors of the innate immune system, NK cells play pivotal roles in immune surveillance and defense against pathogens and tumors. Although whether microwave-mediated NK injury could induce immune suppression, evoke tumorigenesis, and promote tumor progression is still unexplored, proper protective precautions should be taken in microwave-exposed environments.
ERK signaling participatedin microwave-induced injury of NK-92 cells via regulation of cellular apoptosis and perforin expression. However, the mechanisms of microwave-induced injury of NK cells in vivo will be much more complex, and we will establish a corresponding animal model to evaluate the effects.
doi: 10.3967/bes2017.043
-
Abstract:
Objective To investigate microwave-induced morphological and functional injury of natural killer (NK) cells and uncover their mechanisms. Methods NK-92 cells were exposed to 10, 30, and 50 mW/cm2 microwaves for 5 min. Ultrastructural changes, cellular apoptosis and cell cycle regulation were detected at 1 h and 24 h after exposure. Cytotoxic activity was assayed at 1 h after exposure, while perforin and NKG2D expression were detected at 1 h, 6 h, and 12 h after exposure. To clarify the mechanisms, phosphorylated ERK (p-ERK) was detected at 1 h after exposure. Moreover, microwave-induced cellular apoptosis and cell cycle regulation were analyzed after blockade of ERK signaling by using U0126. Results Microwave-induced morphological and ultrastructural injury, dose-dependent apoptosis (P < 0.001) and cell cycle arrest (P < 0.001) were detected at 1 h after microwave exposure. Moreover, significant apoptosis was still detected at 24 h after 50 mW/cm2 microwave exposure (P < 0.01). In the 30 mW/cm2 microwave exposure model, microwaves impaired the cytotoxic activity of NK-92 cells at 1 h and down regulated perforin protein both at 1 h and 6 h after exposure (P < 0.05). Furthermore, p-ERK was down regulated at 1 h after exposure (P < 0.05), while ERK blockade significantly promoted microwave-induced apoptosis (P < 0.05) and downregulation of perforin (P < 0.01). Conclusion Microwave dose-dependently induced morphological and functional injury in NK-92 cells, possibly through ERK-mediated regulation of apoptosis and perforin expression. -
Key words:
- Microwave /
- Natural killer cells /
- Cytotoxicity /
- Apoptosis /
- Cell cycle /
- Perforin
注释:1) Authors' contributions: 2) CONFLICT OF INTEREST: -
Figure 1. Microwave-induced morphological and functional injury of NK-92 cells. (A) NK-92 cells were exposed to 30 mW/cm2 microwaves, the ultrastructure of NK-92 cells was also observed at 1 h and 24 h after exposure by transmission electron microscopy (TEM). (B) NK-92 cells were exposed to 0 (Sham), 10, 30, and 50 mW/cm2 microwaves. One hour after exposure, NK-92 cells were collected and co-cultured with K562 cells at ratio of 10:1 for 4 h. Then, the lactate dehydrogenase (LDH) level in media was analyzed and the percentage of dead cells was calculated. **, P < 0.01 vs. Sham group.
Figure 2. Microwave-induced cellular apoptosis and cell cycle arrest. NK-92 cells were exposed to 0 (Sham), 10, 30, and 50 mW/cm2 microwaves. At 1 h and 24 h after exposure, cells were collected and labeled with Annexin-V-FITC and PI. Then, cellular apoptosis was analyzed by flow cytometry. The representative images after 30 mW/cm2 microwave exposure are shown in (A). And the quantification of apoptosis rates is presented in (B). To confirm the mechanisms of microwave-induced apoptosis, the activity of NK cells was detected at 0.5 h, 1 h, 6 h, and 12 h after microwave exposure by using the Caspase-3 activity kit according to the manufacturer's protocol (C). For analysis of the cell cycle, cells were collected at 1 and 24 h after exposure, and fixed in 70% ethanol at -20 ℃ for 24 h. Then, cells were processed for cell cycle analysis by using flow cytometry. The representative images after 30 mW/cm2 microwave exposure are shown in (D). The percentages of cells in G0/G1 phase, in G2/M phase, and in S phase are shown in (E), (F), and (G), respectively. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. sham group at the same time point after exposure.
Figure 3. Microwaves regulate the expression of perforin and NKG2D in NK-92 cells. NK-92 cells were exposed to 0 (Sham) and 30 mW/cm2 microwaves. Protein was extracted from cells collected at 1 h, 6 h, and 12 h after exposure. The protein expression of perforin and NKG2D was analyzed by western-blotting (A) and the corresponding results of quantification are shown in (B) and (C). *, P < 0.05, **, P < 0.01 vs. sham group at the same time points after exposure. Cells were collected at 1 h, 6 h, and 12 h after exposure, and total RNA was isolated by using TRIzol reagent. After cDNA was synthesized, the mRNA expression of perforin (D) and NKG2D (E), an activating receptor on NK cells, were measured by quantitative reverse transcription polymerase chain reaction (RT-PCR).
Figure 4. ERK signaling participates in microwave-induced injury of NK-92 cells. NK-92 cells were exposed to 0 (Sham) and 30 mW/cm2 microwaves. At 1 h, 6 h, and 12 h after exposure, cells were collected, and the expression of phosphorylated ERK (p-ERK) was detected by western-blotting. Then, p-ERK expression was normalized to total ERK (A). *, P < 0.05 vs. sham group at 1 h after exposure. To blocking ERK signaling, U0126 (a specific ERK inhibitor) was added to NK-92 cells 30 min before exposure at a concentration of 50 μmol/L. One hour after exposure to 0 (Sham) and 30 mW/cm2 microwaves, the level of p-ERK was confirmed by western-blotting and normalized to ERK (B), and the expression of perforin was also measured by western-blotting and normalized to GAPDH (C). The quantitative results of p-ERK and perforin are also shown. *, P < 0.05, **, P < 0.01 vs. sham group; ##, P < 0.01 vs. microwave exposure group (MW). Thirty minutes before microwave exposure, 50 μmol/L U0126 were added to NK-92 cells, using DMSO as a negative control. At 1 h, 6 h, 12 h, and 24 h after 0 and 30 mW/cm2 microwave exposure, cells were collected and used to analyze the cellular apoptosis and cell cycle by using flow cytometry. The apoptosis rates are shown in (D), and the percentage of cells in G0/G1 phase was calculated and displayed in (E). *, P < 0.05, **, P < 0.01, ***, P < 0.001 vs. sham group at the same time point; ##, P < 0.01, ###, P < 0.001 vs. microwave exposure group (MW) at the same time point.
-
[1] Zhao L, Peng RY, Wang SM, et al. Relationship between cognition function and hippocampus structure after long-term microwave exposure. Biomed Environ Sci, 2012; 25, 182-8. https://www.researchgate.net/publication/257295558_Relationship_between_Cognition_Function_and_Hippocampus_Structure_after_Long-term_Microwave_Exposure [2] Ye J, Yao K, Zeng Q, et al. Changes in gap junctional intercellular communication in rabbits lens epithelial cells induced by low power density microwave radiation. Chin Med J, 2002; 115, 1873-6. https://www.researchgate.net/publication/8053020_Changes_in_gap_junctional_intercellular_communication_in_rabits_lens_epithelial_cells_induced_by_low_power_density_microwave_radiation [3] Muller J, Hadeler KP, Muller V, et al. Influence of low power cm-/mm-microwaves on cardiovascular function. Int J Environ Health Res, 2004; 14, 331-41. doi: 10.1080/09603120400004006 [4] Kim YA, Fomenko BS, Agafonova TA, et al. Effects of microwave radiation (340 and 900 MHz) on different structural levels of erythrocyte membranes. Bioelectromagnetics, 1985; 6, 305-12. doi: 10.1002/(ISSN)1521-186X [5] Zhao L, Yang YF, Gao YB, et al. Upregulation of HIF-1alpha via activation of ERK and PI3K pathway mediated protective response to microwave-induced mitochondrial injury in neuron-like cells. Mol Neurobiol, 2014; 50, 1024-34. doi: 10.1007/s12035-014-8667-z [6] Zhao L, Sun C, Xiong L, et al. MicroRNAs: Novel Mechanism Involved in the Pathogenesis of Microwave Exposure on Rats' Hippocampus. J Mol Neurosci: MN, 2014; 53, 222-30. doi: 10.1007/s12031-014-0289-4 [7] Zhang X, Gao Y, Dong J, et al. The compound Chinese medicine "Kang Fu Ling" protects against high power microwave-induced myocardial injury. PloS One, 2014; 9, e101532. doi: 10.1371/journal.pone.0101532 [8] Liu YQ, Gao YB, Dong J, et al. Pathological changes in the sinoatrial node tissues of rats caused by pulsed microwave exposure. Biomed Environ Sci, 2015; 28, 72-5. https://www.researchgate.net/publication/270655734_Pathological_Changes_in_the_Sinoatrial_Node_Tissues_of_Rats_Caused_by_Pulsed_Microwave_Exposure [9] Xiong L, Sun CF, Zhang J, et al. Microwave exposure impairs synaptic plasticity in the rat hippocampus and PC12 cells through over-activation of the NMDA receptor signaling pathway. Biomed Environ Sci, 2015; 28, 13-24. http://www.cnki.com.cn/Article/CJFDTotal-SWYX201501002.htm [10] Wang LF, Li X, Gao YB, et al. Activation of VEGF/Flk-1-ERK Pathway Induced Blood-Brain Barrier Injury After Microwave Exposure. Mol Neurobiol, 2014. https://www.researchgate.net/publication/265418740_Activation_of_VEGFFlk-1-ERK_Pathway_Induced_Blood-Brain_Barrier_Injury_After_Microwave_Exposure [11] Zuo H, Lin T, Wang D, et al. RKIP Regulates Neural Cell Apoptosis Induced by Exposure to Microwave Radiation Partly Through the MEK/ERK/CREB Pathway. Mol Neurobiol, 2014. https://www.researchgate.net/publication/264632036_RKIP_Regulates_Neural_Cell_Apoptosis_Induced_by_Exposure_to_Microwave_Radiation_Partly_Through_the_MEKERKCREB_Pathway [12] Khomenko AG, Sigaev AT, Chukanov VI, et al. Effects of millimetric electromagnetic waves on regional blood flow and effectiveness of multimodal therapy of patients with pulmonary tuberculosis. Vestn Ross Akad Med Nauk, 1995; 42-4. https://www.ncbi.nlm.nih.gov/pubmed/7670342 [13] Rojavin MA, Ziskin MC. Electromagnetic millimeter waves increase the duration of anaesthesia caused by ketamine and chloral hydrate in mice. Int J Radiat Biol, 1997; 72, 475-80. doi: 10.1080/095530097143248 [14] Zaporozhan VN, Khait OV, Bespoyasnaya VV. Application of short-wave therapy in complex treatment for endometrial cancer. Eur J Gynaecol Oncol, 1993; 14, 296-301. https://www.ncbi.nlm.nih.gov/pubmed/8344323 [15] Sambucci M, Laudisi F, Nasta F, et al. Prenatal exposure to non-ionizing radiation: effects of WiFi signals on pregnancy outcome, peripheral B-cell compartment and antibody production. Radiat Res, 2010; 174, 732-40. doi: 10.1667/RR2255.1 [16] Sambucci M, Laudisi F, Nasta F, et al. Early life exposure to 2. 45GHz WiFi-like signals: effects on development and maturation of the immune system. Prog Biophys Mol Biol, 2011; 107, 393-8. doi: 10.1016/j.pbiomolbio.2011.08.012 [17] Makar VR, Logani MK, Bhanushali A, et al. Effect of cyclophosphamide and 61. 22 GHz millimeter waves on T-cell, B-cell, and macrophage functions. Bioelectromagnetics, 2006; 27, 458-66. doi: 10.1002/(ISSN)1521-186X [18] Adam C, King S, Allgeier T, et al. DC-NK cell cross talk as a novel CD4+ T-cell-independent pathway for antitumor CTL induction. Blood, 2005; 106, 338-44. doi: 10.1182/blood-2004-09-3775 [19] Dmoch A, Moszczynski P. Levels of immunoglobulin and subpopulations of T lymphocytes and NK cells in men occupationally exposed to microwave radiation in frequencies of 6-12 GHz. Med Pr, 1998; 49, 45-9. https://www.researchgate.net/publication/13696024_Levels_of_immunoglobulin_and_subpopulations_of_T_lymphocytes_and_NK_cells_in_men_occupationally_exposed_to_microwave_radiation_in_frequencies_of_6-12_GHz [20] Takaki R, Hayakawa Y, Nelson A, et al. IL-21 enhances tumor rejection through a NKG2D-dependent mechanism. J Immunol, 2005; 175, 2167-73. doi: 10.4049/jimmunol.175.4.2167 [21] Poggi A, Zocchi MR. NK cell autoreactivity and autoimmune diseases. Front Immunol, 2014; 5, 27. https://moh-it.pure.elsevier.com/en/publications/nk-cell-autoreactivity-and-autoimmune-diseases [22] Le Bert N, Gasser S. Advances in NKG2D ligand recognition and responses by NK cells. Immunol Cell Biol, 2014; 92, 230-6. doi: 10.1038/icb.2013.111 [23] Wang H, Peng R, Zhou H, et al. Impairment of long-term potentiation induction is essential for the disruption of spatial memory after microwave exposure. Int J Radiat Biol, 2013; 89, 1100-7. doi: 10.3109/09553002.2013.817701 [24] Peinnequin A, Piriou A, Mathieu J, et al. Non-thermal effects of continuous 2. 45 GHz microwaves on Fas-induced apoptosis in human Jurkat T-cell line. Bioelectrochemistry, 2000; 51, 157-61. doi: 10.1016/S0302-4598(00)00064-7 [25] Gatta L, Pinto R, Ubaldi V, et al. Effects of in vivo exposure to GSM-modulated 900 MHz radiation on mouse peripheral lymphocytes. Radiation Research, 2003; 160, 600-5. doi: 10.1667/RR3078 [26] Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science, 1999; 285, 727-9. doi: 10.1126/science.285.5428.727 [27] Yang HK, Cain CA, Lockwood J, et al. Effects of microwave exposure on the hamster immune system. Ⅰ. Natural killer cell activity. Bioelectromagnetics, 1983; 4, 123-39. doi: 10.1002/(ISSN)1521-186X [28] Deschaux P, Douss T, Santini R, et al. Effect of microwave irradiation (2450 MHz) on murine cytotoxic lymphocyte and natural killer (NK) cells. J Microw Power, 1984; 19, 107-10. doi: 10.1080/16070658.1984.11689356 [29] Smialowicz RJ, Rogers RR, Garner RJ, et al. Microwaves (2, 450 MHz) suppress murine natural killer cell activity. Bioelectromagnetics, 1983; 4, 371-81. doi: 10.1002/(ISSN)1521-186X [30] Chang CJ, Chen YY, Lu CC, et al. Ganoderma lucidum stimulates NK cell cytotoxicity by inducing NKG2D/NCR activation and secretion of perforin and granulysin. Innate immunity, 2014; 20, 301-11. doi: 10.1177/1753425913491789 [31] Wu L, Zhang C, Zhang J. HMBOX1 negatively regulates NK cell functions by suppressing the NKG2D/DAP10 signaling pathway. Cell Mol Immunol, 2011; 8, 433-40. doi: 10.1038/cmi.2011.20 [32] Bambard ND, Mathew SO, Mathew PA. LLT1-mediated activation of IFN-gamma production in human natural killer cells involves ERK signalling pathway. Scand J Immunol, 2010; 71, 210-9. doi: 10.1111/sji.2010.71.issue-3 [33] Chen Y, Wang Y, Zhuang Y, et al. Mifepristone increases the cytotoxicity of uterine natural killer cells by acting as a glucocorticoid antagonist via ERK activation. PloS one, 2012; 7, e36413. doi: 10.1371/journal.pone.0036413 [34] Meng M, Li C, Chen D, et al. Novel synthetic immunostimulators with a thiazolidin-4-one ring promote the cytotoxicity of human NK cells via ERK1/2 activation in vitro. Int Immunopharmacol, 2013; 15, 655-60. doi: 10.1016/j.intimp.2013.02.019 [35] Yu MA, Liang P, Yu XL, et al. Multiple courses of immunotherapy with different immune cell types for patients with hepatocellular carcinoma after microwave ablation. Exp Ther Med, 2015; 10, 1460-6. doi: 10.3892/etm.2015.2681?text=abstract [36] Li L, Wang W, Pan H, et al. Microwave ablation combined with OK-432 induces Th1-type response and specific antitumor immunity in a murine model of breast cancer. J Transl Med, 2017; 15, 23. doi: 10.1186/s12967-017-1124-9 -




 下载:
下载:



 Quick Links
Quick Links