-
Iodine is an element that is essential for the synthesis of thyroid hormones. Adequate intake of dietary iodine has been recognized as a critical factor for maintaining health[1]. It is a well-known fact that iodine deficiency can impede the production of thyroid hormones in both the mother and fetus, which increases the risk of brain damage in the fetal stage. Excess iodine can also result in a series of thyroid dysfunction symptoms such as goiter and hypothyroidism. Since approximately 90% of dietary iodine intake is excreted in the urine, the concentration of urinary iodine (UIC) primarily reflects the iodine status. However, a high variability in UIC exists in the day-to-day dietary iodine intake and the large daily flux[2]. Currently, the World Health Organization (WHO) recommends using median spot UIC, but without a specified time, to describe the iodine status[3]. Spot UIC can exhibit fluctuations due to several external factors, including personal habits, environmental conditions, and internal factors. Therefore, the urinary iodine/creatinine (UIC/Cr) ratio in spot urine specimens is often used to describe the iodine status by adjusting the differences in urine volume and sample dilution.
The 24-h urinary iodine excretion (24-h UIE) has been recognized as the gold standard for assessing the iodine status[4]. Obtaining a complete collection of 24-h urine specimens is impractical due to the considerable burden. Consequently, a concise, alternative method for estimating the iodine status in the population based on spot urine and 24-h urine specimens has been increasingly considered. The method for estimating 24-h UIE (est_24-UIE) is obtained using the UIC/Cr ratio in spot urine, multiplied by the estimated 24-h urinary creatinine excretion (est_24-h UCrE)[5]. In fact, the formula for calculating the est_24-h UCrE has already been established in the Japanese and the USA populations, but its applicability to the Chinese population remains unclear. More remarkably, the formula for calculating the est_24-h UCrE has not yet been established in the Chinese population.
Pregnant women comprise a susceptible group of population exhibiting the greatest variations in their physiological metabolism. The status of iodine nutrition is pivotal to maintain the fitness of pregnant women and their fetus during the gestation period. Therefore, the aim of the present study was to develop a more reliable and widely practical method for assessing the iodine status among pregnant women.
This study was performed in Peking Union Medical College Hospital in China from March to November in 2016. All the study subjects were required to have no previous history of thyroid diseases and medication intake (including iodine supplements). A total of 118 pregnant women were finally recruited in this study. The study protocol was approved by the Medical Ethics Committee of the National Institute of Nutrition and Health of the Chinese Center for Disease Control and Prevention. Written informed consent was obtained from every subject. Body height and weight were measured in duplicate using a calibrated stadiometer and a beam scale, respectively. Height was measured to the nearest 0.1 cm, and weight was measured to the nearest 0.1 kg. All subjects were asked to collect their morning spot urine (8:00-12:00 am) and the following 24-h urine specimens. The 24-h urine specimens were measured, and two 10-mL aliquots were obtained and stored at -10 ℃ until analysis.
The UIC was determined using the Sandell-Kolthoff method in the National Reference Laboratory for Iodine Deficiency Disorders[6]. Three levels of certified reference human urine (GBW09108, GBW09110, and GBW0911; National Reference Laboratory for Iodine Deficiency Disorders, Beijing) were applied to ensure the accuracy of the method. The CVs were calculated as 0.3%-4.0% in this study. Urinary creatinine concentration (Cr) was measured using the kinetic Jaffé procedure[7]. Since previous research[8] postulates a suite of hypotheses that the measured 24-h UCrE is relatively proportional to the estimated 24-h UCrE according to the formula and the spot UIC/Cr ratio is positively associated with the 24-h urine UIC/Cr ratio, the estimated 24-h UIE can be calculated using the spot UIC/Cr ratio, multiplied by the estimated 24-h UCrE.
The SAS statistical software (version 9.2, SAS Inc., Cary, NC) was used for data analysis in this study. Age, height, weight, and BMI were expressed as mean ± SD. Since the UIC and Cr/I ratio were not normally distributed, these data were expressed as median values with the 25th and 75th percentiles. Differences in age, height, and 24-h urinary volume among the trimesters of gestation were evaluated using one-way analysis of variance. Differences in the UIC and UIC/Cr ratio between morning spot urine and 24-h urine specimens were analyzed by Mann-Whitney U tests, and the differences among the three trimesters were evaluated using Kruskal-Wallis H tests. A linear regression formula for calculating the est_24-h UCrE, including age, gestation period, height, weight, and BMI, was further developed to calculate the 24-h UIE. The measured values and the estimated values calculated by the formulas were compared[5, 8, 9]. All the values with P < 0.05 were considered to be statistically significant.
The characteristics of the pregnant women are shown in Table 1. During pregnancy, there was a physiological increase in the weight and BMI of the women. However, there were no significant differences in age, height, and 24-h urinary volume between the trimesters of gestation (P > 0.05). The 24-h UIC was significantly lower than spot UIC (P < 0.05), whereas the UIC/Cr ratio in the 24-h urine specimens was significantly higher (P < 0.05). The 24-h UCrE was about 1.10 g/day, ranging from 1.07 to 1.13 g/day, but there was no significant difference during pregnancy (P > 0.05). Similarly, the 24-h UIE was 237 μg/day, ranging from 218 to 245 μg/day, with no significant difference (P > 0.05).
Table 1. Characteristics of Pregnant Women during the Gestation Period
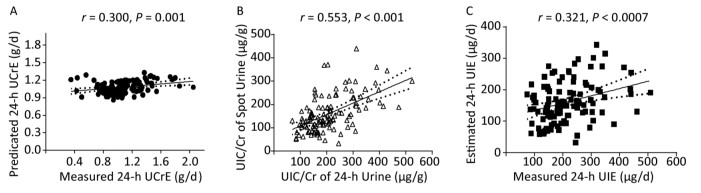
Variables First Trimester Second Trimester Third Trimester All Number (n) 23 51 44 118 Age (years) 31.2 ± 4.3 33.1 ± 4.3 33.6 ± 4.1 33.3 ± 4.2 Height (cm) 164 ± 5 164 ± 4 162 ± 4 163 ± 4 Weight (kg) 59.0 ± 8.8 63.6 ± 8.8 67.8 ± 8.6 64.3 ± 9.2 BMI (kg/m2) 21.8 ± 3.4 23.8 ± 3.2 25.8 ± 3.4 24.2 ± 3.6 Urinary volume (L/24 h) 2.0 (1.3-2.3) 1.7 (1.4-2.4) 1.7 (1.3-2.8) 1.8 (1.3-2.2) 24-h UIC (μg/L) 112 (96-146) 113 (91-149) 131 (100-180) 118 (92-158) Spot UIC (μg/L) 124 (112-157) 141 (93-202) 135 (84-192) 137 (93-193) 24-h UIC/Cr (μg/g) 181 (145-245) 194 (154-295) 204 (145-246) 194 (148-272) Spot UIC/Cr (μg/g) 144 (85-185) 150 (109-221) 149 (116-235) 148 (109-208) 24-h UCrE (g/d) 1.07 (0.95-1.23) 1.04 (0.85-1.22) 1.06 (0.92-1.37) 1.06 (0.88-1.25) 24-h UIE (μg/d) 186 (153-245) 221 (148-299) 212 (153-279) 207 (150-286) Note. Age, Height, Weight, and BMI are expressed as mean ± SD, and urinary volume, 24-h UIC, spot UIC, 24-h UIC/Cr, spot UIC/Cr, 24-h UcrE, and 24-h UIE are expressed as median (25th-75th). Differences in the overall UIC and UIC/Cr ratio or each trimester between the 24-h urine and the spot urine specimens were analyzed using Mann-Whitney U tests. Differences in the UIC and UIC/Cr ratio in the 24-h urine and spot urine specimens among the three trimesters were evaluated using Kruskal-Wallis H tests. A formula to predict 24-h UCrE was also established in this study. The correlation coefficient (R) of the UIC/Cr ratio between the 24-h urine and the spot urine specimens was calculated as 0.553, which is depicted in Figure 1. As independent variables, we sequentially included age, gestation period, height, weight, and BMI in the model. However, significance was observed when only weight was included in the model (P < 0.05). The formula is listed as follows: est_24-h UcrE (g) = 0.011 × weight (kg) + 0.377.

Figure 1. Association of predicted 24-h UCrE and measured 24-h UCrE (A), UIC/Cr of Spot Urine and UIC/Cr of 24-h Urine (B), estimated 24-h UIE and measured 24-h UIE (C).
The formula for calculating the est_24-h UCrE was used for the estimation of 24-h UIE in the pregnant women. To assess the accuracy and the precision of the formula at the population level, we further calculated the est_24-h UIE based on the formula for calculating the 24-h UCrE described by Kesteloot & Joosens, Mage, and Tanaka and our formula. We compared the measured 24-h UIE with the est_24-h UIE, the results of which are presented in Table 2. Significant differences were observed in the results obtained from the formulas of the est_24-h UIE (P < 0.0001). We also observed that the est_24-h UIE was seemingly higher than the measured 24-h UIE during pregnancy when calculated using the Mage formula and our formula, but not the formula of Kesteloot & Joosens and Tanaka.
Table 2. Comparison of Measured and Est_24-h UIE Calculated Using the Formula of Kesteloot & Joosens, Mage, and Tanaka and Our Method
Variables Measured 24-h UIE – Estimated 24-h UIE (μg/day) (Measured 24-h UIE / Estimated 24-h UIE) × 100 (%) Kesteloot & Joosens Mage Tanaka Our Formula Kesteloot & Joosens Mage Tanaka Our Formula First trimester 41.8 (-15.9 to 99.5) 74.0 (24.0 to 124.1) 43.4 (-10.1 to 96.8) 69.1 (16.1 to 122.1) 96.1 (64.1 to 128.1) 76.0 (54.7 to 97.3) 92.7 (65.7 to 119.7) 80.0 (55.3 to 104.8) Second trimester 37.8 (3.2 to 72.4) 63.6 (28.1 to 99.1) 30.2 (-6.4 to 66.8) 63.4 (28.8 to 98.1) 97.1 (82.6 to 111.7) 85.8 (72.7 to 98.9) 101.8 (86.2 to 117.4) 85.7 (72.6 to 98.8) Third trimester 27.4 (-5.9 to 60.8) 46.0 (12.2 to 79.8) 13.6 (-20.7 to 47.8) 44.9 (12.5 to 77.2) 98.4 (84.5 to 112.2) 89.7 (76.7 to 102.7) 104.9 (89.8 to 120.0) 89.9 (77.2 to 102.6) All 34.6 (12.9 to 56.4) 58.9 (37.2 to 80.6) 26.3 (4.0 to 48.7) 57.5 (36.3 to 78.8) 97.4 (87.7 to 107.2) 85.5 (77.2 to 93.8) 101.3 (91.4 to 111.2) 86.3 (77.8 to 94.7) In Table 2, we found that the accuracy is relatively excellent by the formula of Kesteloot & Joosens, Tanaka than Mage's and our formula.
In this study, we observed a significant difference in the UIC and UIC/Cr ratio between the spot urine and the 24-h urine specimens. We also calculated the est_24-h UIE and compared it with the measured 24-h UIE to examine the accuracy and the precision of the formula. Taken together, the results showed that the est_24-h UIE derived by the 24-h UCrE using the formula of Kesteloot & Joosens and Tanaka was more similar to the measured 24-h UIE, whereas the 24-h UIE calculated by our formula appeared only to be 80%-90% of the actual 24-h UIE.
It is well known that spot UIC varies throughout the day and from day to day, even under a circadian rhythm. In fact, the results of this study showed that morning spot UIC more easily overestimated the iodine status in the population in comparison with the 24-h UIC. Thereby, the UIC/Cr ratio as urine Cr adjustment has ever been used as a reference comparator for analysis. However, we found that the UIC/Cr ratio in the morning spot urine specimens was significantly higher than the values of actual 24-h urine specimens (P < 0.05). These findings are consistent with the WHO reporting that the UIC/Cr ratio does not appear to be a good proxy for expressing the iodine status in the population[6], but are in contrast to the suggestion by a recent study in China[10]. This might imply that while assessing the iodine status through the UIC/Cr ratio adjustment, the physiological and nutritional parameters should be considered in the future.
In this study, we developed the formula for calculating the est_24-h UCrE; using the est_24-h UIE calculated by our formula of est_24-h UCrE was found to be not more accurate in contrast to the formula of Kesteloot & Joosens and Tanaka. This is due to the fact that there is a bias since the formula for calculating the est_24-h UCrE is derived using a small sample size of pregnant women. Moreover, various factors can also affect urine Cr excretion, including sampling time, strenuous exercise, emotional stress, dietary intake of creatine and creatinine, infection, fever, and trauma. In this study, all the subjects were primarily elderly pregnant women, and hence, representativeness is still a question. Whist it did not using a biochemical labelling method, and the completeness of 24-h urine collection is only according to the record of urine collection time, urine volume, and response to questions about urine collection completeness adapted from questionnaires.
In conclusion, we developed a more reliable, widely practical, and alternative method for assessing the iodine status in pregnant women in China. The formula for calculating the est_24-h UCrE was also established, and the est_24-h UIE can be further calculated as the UIC/Cr ratio in the morning spot urine specimens, multiplied by the est_24-h UCrE. However, compared with the formula of Kesteloot & Joosens and Tanaka, our formula failed to exactly predict the actual 24-h UCrE and the actual 24-h UIE. Thus, the formula for calculating the est_24-h UCrE described by Kesteloot & Joosens and Tanaka could be suggested to be better and temporarily used for assessing the iodine status in pregnant women.
We are extremely grateful to the subjects who participated in this study and to the doctors in the outpatient department of Peking Union Medical College Hospital in Beijing for their support during the recruitment of the subjects. We also wish to express our gratitude to LI Xiu Wei, CAI Jie, and WU Jing Huan for their assistance with experiments.
doi: 10.3967/bes2018.072
Using an Alternative Method to Estimate the Status of Iodine Nutrition in Pregnant Women
-
-
Table 1. Characteristics of Pregnant Women during the Gestation Period
Variables First Trimester Second Trimester Third Trimester All Number (n) 23 51 44 118 Age (years) 31.2 ± 4.3 33.1 ± 4.3 33.6 ± 4.1 33.3 ± 4.2 Height (cm) 164 ± 5 164 ± 4 162 ± 4 163 ± 4 Weight (kg) 59.0 ± 8.8 63.6 ± 8.8 67.8 ± 8.6 64.3 ± 9.2 BMI (kg/m2) 21.8 ± 3.4 23.8 ± 3.2 25.8 ± 3.4 24.2 ± 3.6 Urinary volume (L/24 h) 2.0 (1.3-2.3) 1.7 (1.4-2.4) 1.7 (1.3-2.8) 1.8 (1.3-2.2) 24-h UIC (μg/L) 112 (96-146) 113 (91-149) 131 (100-180) 118 (92-158) Spot UIC (μg/L) 124 (112-157) 141 (93-202) 135 (84-192) 137 (93-193) 24-h UIC/Cr (μg/g) 181 (145-245) 194 (154-295) 204 (145-246) 194 (148-272) Spot UIC/Cr (μg/g) 144 (85-185) 150 (109-221) 149 (116-235) 148 (109-208) 24-h UCrE (g/d) 1.07 (0.95-1.23) 1.04 (0.85-1.22) 1.06 (0.92-1.37) 1.06 (0.88-1.25) 24-h UIE (μg/d) 186 (153-245) 221 (148-299) 212 (153-279) 207 (150-286) Note. Age, Height, Weight, and BMI are expressed as mean ± SD, and urinary volume, 24-h UIC, spot UIC, 24-h UIC/Cr, spot UIC/Cr, 24-h UcrE, and 24-h UIE are expressed as median (25th-75th). Differences in the overall UIC and UIC/Cr ratio or each trimester between the 24-h urine and the spot urine specimens were analyzed using Mann-Whitney U tests. Differences in the UIC and UIC/Cr ratio in the 24-h urine and spot urine specimens among the three trimesters were evaluated using Kruskal-Wallis H tests. Table 2. Comparison of Measured and Est_24-h UIE Calculated Using the Formula of Kesteloot & Joosens, Mage, and Tanaka and Our Method
Variables Measured 24-h UIE – Estimated 24-h UIE (μg/day) (Measured 24-h UIE / Estimated 24-h UIE) × 100 (%) Kesteloot & Joosens Mage Tanaka Our Formula Kesteloot & Joosens Mage Tanaka Our Formula First trimester 41.8 (-15.9 to 99.5) 74.0 (24.0 to 124.1) 43.4 (-10.1 to 96.8) 69.1 (16.1 to 122.1) 96.1 (64.1 to 128.1) 76.0 (54.7 to 97.3) 92.7 (65.7 to 119.7) 80.0 (55.3 to 104.8) Second trimester 37.8 (3.2 to 72.4) 63.6 (28.1 to 99.1) 30.2 (-6.4 to 66.8) 63.4 (28.8 to 98.1) 97.1 (82.6 to 111.7) 85.8 (72.7 to 98.9) 101.8 (86.2 to 117.4) 85.7 (72.6 to 98.8) Third trimester 27.4 (-5.9 to 60.8) 46.0 (12.2 to 79.8) 13.6 (-20.7 to 47.8) 44.9 (12.5 to 77.2) 98.4 (84.5 to 112.2) 89.7 (76.7 to 102.7) 104.9 (89.8 to 120.0) 89.9 (77.2 to 102.6) All 34.6 (12.9 to 56.4) 58.9 (37.2 to 80.6) 26.3 (4.0 to 48.7) 57.5 (36.3 to 78.8) 97.4 (87.7 to 107.2) 85.5 (77.2 to 93.8) 101.3 (91.4 to 111.2) 86.3 (77.8 to 94.7) -
[1] Pearce EN, Lazarus JH, Moreno-Reyes R, et al. Consequences of iodine deficiency and excess in pregnant women:an overview of current knowns and unknowns. Am J Clin Nutr, 2016; 104, 918-23. doi: 10.3945/ajcn.115.110429 [2] Rasmussen LB, Ovesen L, Christiansen E. Day-to-day and within-day variation in urinary iodine excretion. Eur J Clin Nutr, 1999; 53, 401-7. doi: 10.1038/sj.ejcn.1600762 [3] WHO. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination. A Guide for Programme Managers. Geneva:WHO, 2007. [4] Remer T, Fonteyn N, Alexy U, et al. Longitudinal examination of 24-h urinary iodine excretion in schoolchildren as a sensitive, hydration status-independent research tool for studying iodine status. Am J Clin Nutr, 2006; 83, 639-46. doi: 10.1093/ajcn.83.3.639 [5] Kawasaki T, Itoh K, Uezono K, et al. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin Exp Pharmacol Physiol, 1993; 20, 7-14. doi: 10.1111/cep.1993.20.issue-1 [6] Dunn JT, Crutchfield HE, Gutekunst R, et al. Two simple methods for measuring iodine in urine. Thyroid, 1993; 3, 119-23. doi: 10.1089/thy.1993.3.119 [7] Kesteloot H, Joossens J. On the determinants of the creatinine clearance:a population study. J Hum Hypertens, 1996; 10, 245-9. http://europepmc.org/abstract/MED/8736456 [8] Tanaka T, Okamura T, Miura K, et al. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens, 2002; 16, 97-103. doi: 10.1038/sj.jhh.1001307 [9] Mage DT, Allen RH, Kodali A. Creatinine corrections for estimating children's and adult's pesticide intake doses in equilibrium with urinary pesticide and creatinine concentrations. J Expo Sci Environ Epidemiol, 2007; 18, 360. http://europepmc.org/abstract/MED/17878925 [10] Li CY, Peng S, Zhang XM, et al. The urine iodine to creatinine as an optimal index of iodine during pregnancy in an iodine adequate area in China. J Clin Endocr Metab, 2016; 101, 1290-8. doi: 10.1210/jc.2015-3519 -




 下载:
下载:



 Quick Links
Quick Links