-
Phthalates are multifunctional compounds that are used in various commercial and industrial products, such as food packagings, toys, cosmetics, building materials, and personal care products[1-3], Di(2-ethylhexyl) phthalate (DEHP), one of the most commonly used phthalates, is ubiquitously present in humans[4]. Studies have shown that DEHP has some potentially adverse effects on health, including abnormal timing of parturition, reproductive dysfunction, and low birth weight[5-7]. Some authors reported that DEHP affects the function of the hypothalamus-pituitary-ovarian axis and influences the mRNA and protein levels of GnRHR in the pituitary[8]. Furthermore, limited studies have shown that DEHP affects oocyte maturation by modulating the expression of luteinizing hormone receptor (LHR)[9-10]. In this study, we explored the effects of DEHP exposure on ovarian histology and ultra-structure, and then we studied the possible involvement of oncogenes and apoptosis-related genes mechanisms after DEHP treatment were studied, then we attempted to explore the role of LHR and GnRHR in the ovary.
A total of 40 healthy 35-day-old female Sprague-Dawley rats weighing approximately 60.37 ± 5.55 g were obtained from Guangdong Provincial Medical Experimental Animal Center. All rats were maintained in cages under a 12-h light-dark cycle with controlled ambient temperature (18-23 ℃) and humidity (50%-70%) and provided with free access to commercial rodent chow and tap water. All rats were allowed to acclimatize for one week upon arrival. All animal treatments were approved by the Shenzhen Center for Disease Control and Prevention Animal Care and Use Committee. We randomly divided the rats into four groups and exposed each group to a different concentration of DEHP (0, 100, 500, and 1, 500 mg/kg body weight/day) for six weeks (five days per week) by gavage. DEHP was dissolved in corn oil. Rat body weight was measured to determine the dosage of DEHP every two days.
Rat ovarian tissues were fixed in 4% paraformaldehyde solution at pH 7.4 for at least 48 h. Once all ovarian samples were fixed, they were subjected to dehydration, clarification, and inclusion. Hydrated and deparaffinized sections were stained with hematoxylin and eosin (HE) for histologic observation. In addition, rat ovarian tissues were fixed with 2.5% glutaraldehyde for at least 12 h at 4 ℃, rinsed in phosphate-buffered saline (PBS), fixed in 1% osmium tetroxide solution for 1 h at 4 ℃, dehydrated in a series of ethanol, and then embedded in epoxy resin. Next, ultrathin sections of the ovarian tissues were obtained, stained with uranyl acetate and lead citrate, and then observed by using a transmission electron microscope (HITACHI H-7650) for the ultra-structure study.
RNA isolation from ovarian tissues and quantitative RT-PCR of apoptosis genes were performed For western blotting analysis, ovarian tissues were homogenized and after electrophoresis, the proteins were then transferred into PVDF membrane. Next, mouse anti-β-actin, rabbit anti- luteinizing hormone receptor (LHR), and rabbit anti-GnRHR antibodies were diluted in blocking buffer and incubated at 4 ℃ for 12 h. Anti-rabbit and anti-mouse IgG secondary antibodies were diluted to 1:4, 000 in Tris-HCl buffer solution+Tween (TBST) and incubated at 25 ℃ for 60 min. The proteins were then incubated with horseradish peroxidase- conjugated secondary antibodies and visualized using ECL substrate and an Image Quant RT ECL system [GE Healthcare (USA)].
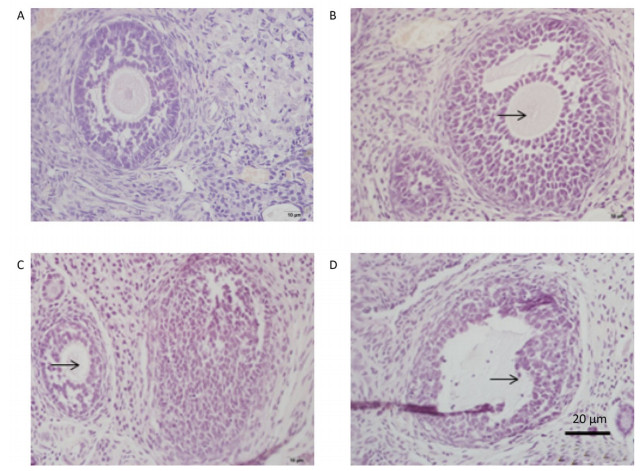
Oocyte in the primary follicle disappeared after treatment with 100 mg/kg DEHP (Figure 1B). In the group treated with 500 mg/kg DEHP, oocyte loss occurred in the primary follicle, along with loose structure and detachment of granular cells, as well as the presence of follicular atresia (Figure 1C). In the group treated with 1, 500 mg/kg DEHP, oocyte loss was observed in the primary follicle, and malalignment of granular cells and fuzziness of the zona pellucida were also observed (Figure 1D).

Figure 1. Photomicrographs of a HE-stained paraffin section of rat ovary (n = 5). (A) the control group. (B) the 100 mg/kg DEHP group. (C) the 500 mg/kg DEHP group. (D) the 1, 500 mg/kg DEHP group.
Our observation results showed that the ultra-structure of granule cells was changed significantly after treatment with DEHP. In the group treated with 100 mg/kg DEHP, although most of the granule cells were normal in appearance, the individual cells showed indistinction of the nuclear membrane, cell death, swelling of the mitochondria, and vacuolation of cytoplasm (Figure 2B). In the group treated with 500 mg/kg DEHP, mitochondrial crest fracture and swelling of the mitochondria were found in granular hemocytes (Figure 2C). In the group treated with 1, 500 mg/kg DEHP, granule cells varied in size and shape. Large nucleus, increasing ratio of nuclear pulp, low number and swelling of mitochondria, reduction of mitochondrial cristae and organelles, and pathological changes of cell degeneration and necrosis were observed in granule cells (Figure 2D).
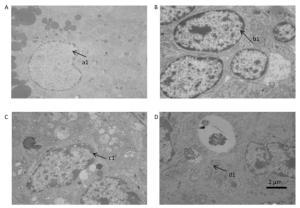
Figure 2. Transmission electron microscopy results revealed the ovarian ultrastructure (n = 5). (A) the control group. (B) 100 mg/kg the DEHP group. (C) the 500 mg/kg DEHP group. (D) the 1, 500 mg/kg DEHP group.
The expression of the apoptosis-related gene Bcl-2 was downregulated (P < 0.05) after treatment with 1, 500 mg/kg DEHP, compared to that in the control group, and showed no significant difference with that in the groups treated with 100 mg/kg and 500 mg/kg DEHP groups (P > 0.05). Caspase-3 and caspase-8 expression was significantly upregulated after treatment with different doses of DEHP. Caspase-9 expression was significantly increased after treatment with 1, 500 mg/kg DEHP, compared to that in the control group (P < 0.05). In addition, k-ras expression was upregulated in the 500 mg/kg and 1, 500 mg/kg DEHP groups, compared to that in the control group (P < 0.01 and P < 0.01).
The protein levels of LHR and GnRHR in rat ovary were measured by western blotting. The expression of LHR and GnRHR proteins did not significantly change after treatment with 100 mg/kg DEHP, compared to that in the control group (P > 0.05) (Figure 3). In the groups treated with 500 mg/kg and 1, 500 mg/kg DEHP, the expresion level of LHR was significantly downregulated by 25%-35%, compared to that in the control group (P < 0.05 and P < 0.01) (Figure 3A). The expression level of GnRHR was significantly decreased by 60%-80% after treatment with 500 mg/kg and 1, 500 mg/kg DEHP (P < 0.01) (Figure 3B).
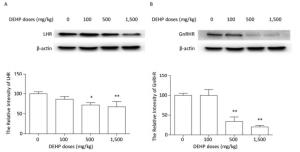
Figure 3. Effects of DEHP on the expression of hormone-related proteins. Values are expressed as mean ± SEM (n = 5). (A) Western blotting analysis results showed the levels of LHR. (B) Western blotting analysis results showed changes in GnRHR level. Significant differences compared to the control group (0 mg/kg DEHP) are indicated at *P < 0.05 and **P < 0.01.
In conclusion, we found that GnRH gene expression in female rats was significantly decreased after DEHP treatment. Thus, we speculated that DEHP could reduce the levels of GnRH and GnRHR. Our results indicated that DEHP might induce ovarian toxicity and reproductive system dysfunction by affecting follicular development and LHR and GnRHR expression.
doi: 10.3967/bes2019.071
Di-2-Ethylhexyl Phthalate Induces Ovarian Toxicity and Alters Protein Expression of Hormone-regulated Receptors in Rats
-
-
Figure 3. Effects of DEHP on the expression of hormone-related proteins. Values are expressed as mean ± SEM (n = 5). (A) Western blotting analysis results showed the levels of LHR. (B) Western blotting analysis results showed changes in GnRHR level. Significant differences compared to the control group (0 mg/kg DEHP) are indicated at *P < 0.05 and **P < 0.01.
-
[1] Komada M, Gendai Y, Kagawa N, et al. Prenatal exposure to di-(2-ethylhexyl) phthalate impairs development of the mouse neocortex. Toxicology Letters, 2016; 259, 69-79. doi: 10.1016/j.toxlet.2016.07.019 [2] Koniecki D, Wang R, Moody RP, et al. Phthalates in cosmetic and personal care products:concentrations and possible dermal exposure. Environ Res, 2011; 111, 329-36. doi: 10.1016/j.envres.2011.01.013 [3] Yongan Wang, Qing Yang, Wei Liu, et al. DEHP exposure in utero disturbs sex determination and is potentially linked with precocious puberty in female mice. Toxicol Appl Pharmacol, 2016; 307, 123-9. doi: 10.1016/j.taap.2016.08.001 [4] Nardelli TC, Albert O, Lalancette C, et al. In utero and lactational exposure study in rats to identify replacements for di(2-ethylhexyl) phthalate. Scientific Reports, 2017; 7, 3862. doi: 10.1038/s41598-017-03979-0 [5] Sant KE, Dolinoy DC, Jilek JL, et al. Mono-2-ethylhexyl phthalatedisrupts neurulation and modifies the embryonic redox environment and gene expression. Reprod Toxicol, 2016; 63, 32-48. doi: 10.1016/j.reprotox.2016.03.042 [6] Hannon PR, Brannick KE, Wang W, et al. Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol Appl Pharmacol, 2015; 284, 42-53. doi: 10.1016/j.taap.2015.02.010 [7] Niermann S, Rattan S, Brehm E, et al. Prenatal exposure to di-(2-ethylhexyl) phthalate (DEHP) affects reproductive outcomes in female mice. Reprod Toxicol, 2015; 53, 23-32. doi: 10.1016/j.reprotox.2015.02.013 [8] Liu T, Li N, Zhu J, et al. Effects of di-(2-ethylhexyl) phthalate on the hypothalamus-pituitary-ovarianaxis in adult female rats. Reprod Toxicol, 2014; 46, 141-7. doi: 10.1016/j.reprotox.2014.03.006 [9] Carnevali O, Tosti L, Speciale C, et al. DEHP impairs zebrafish reproduction by affecting critical factors in oogenesis. PLoS One, 2010; 5, e10201. doi: 10.1371/journal.pone.0010201 [10] Zhang XF, Zhang T, Wang L, et al. Effects of diethylhexyl phthalate (DEHP) given neonatally on spermatogenesis of mice. Mol Biol Rep, 2013; 40, 6509-17. doi: 10.1007/s11033-013-2769-y -




 下载:
下载:



 Quick Links
Quick Links