-
As generally known, many hospitals provide routine care on weekdays but only emergency or urgent care on holidays and weekends. Hospital staffing is numerically reduced on holidays and weekends, as well as the available expertise on duty. The onset of acute myocardial infarction (AMI) on off days was reported to be associated with higher short-term mortality[1,2]. A similar phenomenon was also observed in patients with emergent diseases who were admitted on weekends and in the nighttime, as well as those who received elective procedures on weekends[3-5]. Therefore, the concept of the “weekend effect” (or “off-hours effect”) was raised. That is, patients who are hospitalized during the weekends (or on off-hours) suffer worse outcomes compared with those who are admitted on weekdays (regular work hours) in terms of both mortality and length of hospital stay[5,6]. To date, large data is limited to determine whether this phenomenon also exists in China. Since the management of patients who underwent percutaneous coronary intervention (PCI) requires considerable diagnostic and therapeutic procedures that may not be uniformly available throughout the off days, it is important to address the issue to improve the quality of medical care and hospital management for this population. Therefore, we conducted an association analysis regarding the effect of PCI time on the 2-year outcomes in this population using a large cohort data from a famous cardiovascular center in northern China.
A total of 10,724 consecutive cases with CAD who underwent PCI were enrolled from January to December 2013 in the largest cardiovascular center of China. Off days are China’s legal holidays and weekends. Legal holidays in the year 2013 included New Year’s Day (3 days), Spring Festival (7 days), Qingming Festival (3 days), Labor Day (3 days), Dragon Festival (3 days), Mid-Autumn Festival (3 days), and National Day (7 days). Weekends include Saturday and Sunday, excluding those work-weekends due to holidays swapping. ST-segment elevated myocardial infarction (STEMI), non-ST-segment elevated myocardial infarction (NSTEMI), unstable angina pectoris (UAP), and stable coronary artery disease (SCAD) were diagnosed according to international guidelines. Procedural details and follow-up referred to published studies[7]. The primary endpoint was all-cause death, and secondary endpoints included major adverse cardiac and cerebrovascular events (MACCE), cardiac death, revascularization, MI, stroke, stent thrombosis (ST), and bleeding. MACCE included all-cause death, revascularization, myocardial infarction (MI), and stroke. Cardiac death was identified as death caused by AMI, heart failure, and/or malignant arrhythmia definitely; or death that could not be explained clearly by other reasons. ST included definite, probable, or possible ST based on the Academic Research Consortium criteria. Bleeding was defined according to the criteria established by the Bleeding Academic Research Consortium (BARC), excluding BARC 0 and 1 type.
Among the 10,724 cases analyzed, patients who received PCI on off days and workdays accounted for 1.25% and 98.75%, respectively. In the PCI on off days group, the majority (84.3%) were patients with AMI, which was significantly higher than that in the PCI on workdays group (17.1%, P < 0.001). Accordingly, the majority (80.6%) were patients who received primary PCI, compared with the PCI on workdays group (2.0%, P < 0.001). The average Synergy between PCI with Taxus and the Cardiac Surgery (SYNTAX) score in the PCI on off days group was 14.8 ± 8.6, higher than that in the PCI on workdays group (11.7 ± 8.1, P < 0.001). PCI on off days group had less target lesions (1.29 ± 0.62 and 1.41 ± 0.66, P = 0.037) and stent per patient (1.52 ± 1.05 and 1.81 ± 1.11, P = 0.002), but longer time of procedure (46.7 ± 41.6 and 36.5 ± 31.4, P = 0.006) than that in the PCI on workdays group. Cases in the PCI on off days group had higher body mass index (BMI) (26.7 ± 3.6 and 25.9 ± 3.2, P = 0.014), more prior revascularization history (61.9% and 25.7%, P < 0.001), but lower LVEF level (54.9 ± 8.3 and 62.9 ± 7.2, P < 0.001) than cases in the PCI on workdays group. The use of aspirin, statins, calcium antagonists, and β-blockers were less in the PCI on off days group (93.3% and 98.8%, P < 0.001; 88.8% and 96.0%, P < 0.001; 37.3% and 48.8%, P = 0.008; 84.3% and 90.3%, P = 0.021), while P2Y12 inhibitors were administered similarly between the two groups. The difference in calcium antagonists and β-blockers use between the groups may because, in the PCI on off days group, the majority (84.3%) were patients with AMI, which was significantly higher than that in the PCI on workdays group (17.1%, P < 0.001) (Table 1). The negative inotropic action from calcium antagonists and β-blockers should be cautious in patients with AMI. However, the differences in aspirin and statins use between groups were confused, and we speculate that it is due to patients’ characteristics, such as high bleeding risk or hepatic insufficiency.
Table 1. The baselines clinical, angiographic, procedural characteristics, medication situation and 2-year outcomes between the two groups
Variables All (n = 10,724) PCI on off days (n = 134) PCI on workdays (n = 10,590) P value Demographic characteristics Male gender, n (%) 8,272 (77.1) 105 (78.4) 8,167 (77.1) 0.734 Age, years 58.4 ± 10.3 58.2 ± 11.7 58.4 ± 10.3 0.900 BMI, kg/m2 25.9 ± 3.2 26.7 ± 3.6 25.9 ± 3.2 0.014 Coexisting conditions, n (%) Hypertension 6,906 (64.4) 83 (61.9) 6,823 (64.4) 0.550 DM 3,238 (30.2) 41 (30.6) 3,197 (30.2) 0.919 Hyperlipidemia 7,211 (67.2) 91 (67.9) 7,120 (67.2) 0.868 Previous MI 2,061 (19.2) 24 (17.9) 2,037 (19.2) 0.699 Prior PCI or CABG 2,808 (26.2) 83 (61.9) 2,725 (25.7) < 0.001 Current smoker 6,123 (57.1) 90 (67.2) 6,033 (57.0) 0.018 Family history of CAD 2,651 (24.7) 31 (23.1) 2,620 (24.8) 0.666 CVD 1,150 (10.7) 19 (14.2) 1,131 (10.7) 0.193 PVD 288 (2.7) 5 (3.7) 283 (2.7) 0.451 COPD 247 (2.3) 0 (0.0) 247 (2.3) 0.074 LVEF (%) 62.8 ± 7.4 54.9 ± 8.3 62.9 ± 7.2 < 0.001 Clinical presentation, n (%) Asymptomatic ischemia 869 (8.1) 3 (2.2) 866 (8.2) < 0.001 Stable angina 3,424 (31.9) 5 (3.7) 3,419 (32.3) < 0.001 Unstable angina pectoris 4,509 (42.0) 13 (9.7) 4,496 (42.5) < 0.001 AMI 1,922 (17.9) 113 (84.3) 1,809 (17.1) < 0.001 STEMI 1,447 (13.5) 104 (77.6) 1,343 (12.7) < 0.001 NSTEMI 475 (4.4) 9 (6.7) 466 (4.4) 0.195 Laboratory examination eGFR before PCI, mL·min−1·1.73 m−2 91.3 ± 15.1 89.4 ± 19.4 91.3 ± 15.1 0.256 HGB before PCI, g/L 141.0 ± 15.8 141.0 ± 16.5 141.0 ± 15.8 0.985 PLT before PCI, 109/L 203.6 ± 54.4 199.2 ± 77.0 203.6 ± 54.1 0.348 Uric acid, µmol/L 341.6 ± 84.7 324.5 ± 90.5 341.8 ± 84.6 0.018 HbA1c, % 6.6 ± 1.2 7.0 ± 1.7 6.6 ± 1.2 0.008 LDL-C, mmol/L 2.50 ± 0.90 2.74 ± 0.83 2.50 ± 0.90 0.003 ESR, mm/h 10.8 ± 11.3 10.8 ± 11.0 10.8 ± 11.3 0.988 Angiographic and procedural characteristics SNYTAX score 11.7 ± 8.1 14.8 ± 8.6 11.7 ± 8.1 < 0.001 Residual SNYTAX 3.4 ± 5.7 4.4 ± 6.9 3.4 ± 5.7 0.100 LM or TVD, n (%) 457 (4.3) 2 (1.5) 455 (4.3) 0.110 LAD involved, n (%) 9,702 (90.5%) 124 (92.5) 9,578 (90.4) 0.412 No. of target lesions 1.40 ± 0.66 1.29 ± 0.62 1.41 ± 0.66 0.037 No. of stent per patient 1.80 ± 1.11 1.52 ± 1.05 1.81 ± 1.11 0.002 Time of procedure, min 36.7 ± 31.5 46.7 ± 41.6 36.5 ± 31.4 0.006 Primary PCI, n (%) 323 (3.0) 108 (80.6) 215 (2.0) < 0.001 Procedure and stent type, n (%) 0.079 PTCA 237 (2.2) 7 (5.2) 230 (2.2) BMS 64 (0.6) 2 (1.5) 62 (0.6) First-generation durable polymer DES 597 (5.6) 7 (5.2) 590 (5.6) Second-generation durable polymer DES 6,094 (56.8) 80 (59.7) 6,014 (56.8) Domestic biodegradable polymer DES 1,572 (14.7) 13 (9.7) 1,559 (14.7) Mixed implantation of DES 1,692 (15.8) 19 (14.2) 1,673 (15.8) Others (Janus, Yinyi) 167 (1.6) 4 (3.0) 163 (1.5) Procedure unsuccess 301 (2.8) 2 (1.5) 299 (2.8) Medication, n (%) Aspirin 10,585 (98.7) 125 (93.3) 10,460 (98.8) < 0.001 Clopidogrel 10,701 (99.8) 134 (100.0) 10,567 (99.8) 0.589 Ticagrelor 19 (0.2) 0 (0.0) 19 (0.2) 0.624 DAPT 10,583 (98.7) 125 (93.3) 10,458 (98.8) < 0.001 Statin 10,285 (95.9) 119 (88.8) 10,166 (96.0) < 0.001 Calcium antagonist 5,216 (48.6) 50 (37.3) 5,166 (48.8) 0.008 β-blocker 9,673 (90.2) 113 (84.3) 9,560 (90.3) 0.021 2-year outcomes, n (%) All-cause death 131 (1.2) 6 (4.5) 125 (1.2) 0.001 MACCE 1,295 (12.1) 23 (17.2) 1,272 (12.0) 0.069 Cardiac death 74 (0.7) 4 (3.0) 70 (0.7) 0.001 Myocardial infarction 212 (2.0) 4 (3.0) 208 (2.0) 0.399 Revascularization 923 (8.6) 14 (10.4) 909 (8.6) 0.445 Stent thrombosis 91 (0.8) 4 (3.0) 87 (0.8) 0.007 Stroke 145 (1.4) 2 (1.5) 143 (1.4) 0.887 Bleeding 702 (6.5) 6 (4.5) 696 (6.6) 0.330 Note. AMI, acute myocardial infarction; BMI, body mass index; BMS, bare metal stent; CAD, coronary artery disease; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CVD, cerebral vascular disease; DES, drug-eluting stent; DM, diabetes mellitus; DAPT, dual anti-platelet therapy; eGFR, estimated glomerular filtration rate; ESR, erythrocyte sedimentation rate; HGB, hemoglobin; HbA1c, hemoglobin A1c; LAD, left anterior descending artery; LM, left main; LDL-C, low density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non-ST-segment elevated myocardial infarction; PCI, percutaneous coronary intervention; PLT, platelet; PTCA, percutaneous transluminal coronary angioplasty; PVD, peripheral vascular disease; STEMI, ST-segment elevated myocardial infarction; SYNTAX, Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery; TC, total cholesterol; TVD, three-vessel disease. Data are expressed as mean ± standard deviation; or counts (percentage). Clinical follow-up was completed in 10,665 patients (99.4%) for 2 years. The average follow-up was 872.4 days. The occurrence of adverse cardiovascular events in each group is listed in Table 1. During the 2-year follow-up, the rates of all-cause death, cardiac death, and ST were significantly higher in the PCI on off days group (4.5% and 1.2%, P = 0.001; 3.0% and 0.7%, P = 0.001; 3.0% and 0.8%, P = 0.007). On the other hand, the rates of MACCE, MI, revascularization, stroke, and bleeding were similar between the two groups (all P > 0.05). Kaplan-Meier survival curves showed that all-cause death, cardiac death, ST, and MACCE cumulative event rates were all significantly higher in the PCI on off days group compared with the PCI on workdays group (Figure 1).
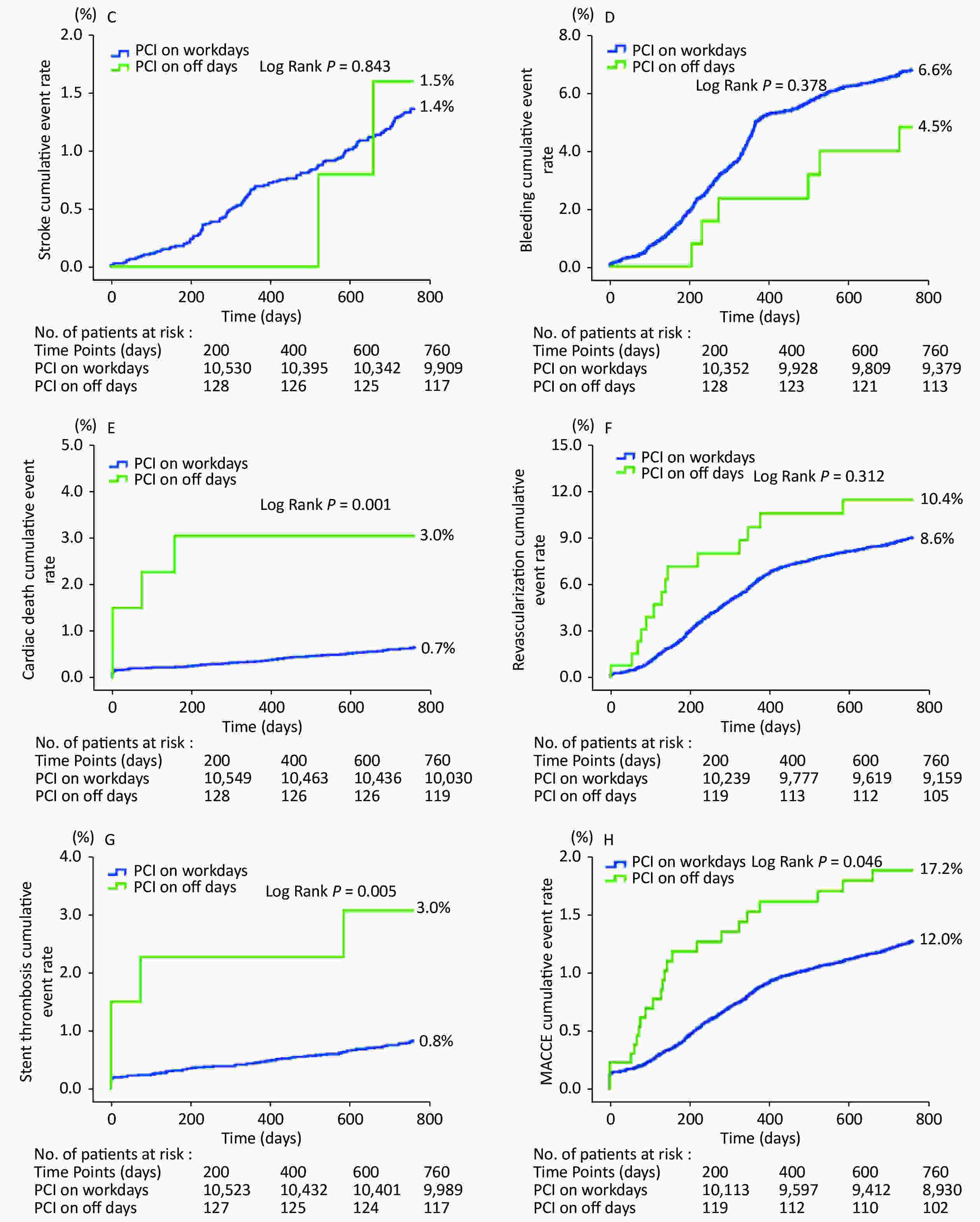
Figure 1. Kaplan-Meier survival curves. (A) all-cause death; (B) myocardial infarction; (C) stroke; (D) bleeding; (E) cardiac death; (F) revascularization; (G) stent thrombosis; (H) major adverse cardiac and cerebrovascular events.
Cox regression models indicated that: 1) In the unadjusted Cox regression model (model 2) and multivariate Cox regression model, which only adjusted for age, sex and BMI (model 3), patients who received PCI on off days were significantly associated with higher risks of all-cause death, cardiac death, and MACCE compared with cases in the PCI on workdays group (model 2: HR 3.93, 95% CI 1.73–8.92, P = 0.001; HR 4.67, 95% CI 1.70–12.78, P = 0.003; HR 1.52, 95% CI 1.004–2.29, P = 0.048) (model 3: HR 4.22, 95% CI 1.86–9.59, P = 0.001; HR 4.87, 95% CI 1.77–13.39, P = 0.002; HR 1.52, 95% CI 1.009–2.30, P = 0.045); 2) In the multivariate Cox regression models, which adjusted for demographic variables (age, sex, BMI) and severity variables (model 4), or adjusted for demographic variables (age, sex, BMI), medical management variables, and invasive management variables (model 5), statistical significance was diminished; 3) In the multivariate Cox regression model, which adjusted for demographic variables (age, sex, BMI) and all the variables showing significant differences between the two groups (model 1), being likely confounders, statistical significance was also diminished (P > 0.05). (Tables 2, 3)
Table 2. HRs of events within 2 years of CAD patients who received PCI on off days or workdays in model 1
Item HR (95% CI) P value Wald value All-cause death 0.91 (0.34, 2.43) 0.854 0.034 MACCE 0.86 (0.53, 1.39) 0.539 0.378 Cardiac death 0.77 (0.24, 2.48) 0.658 0.195 Myocardial infarction 0.64 (0.21, 1.94) 0.431 0.619 Revascularization 0.91 (0.49, 1.68) 0.765 0.089 Stent thrombosis 1.36 (0.39, 4.71) 0.629 0.233 Stroke 0.50 (0.11, 2.28) 0.367 0.814 Bleeding 1.00 (0.40, 2.49) 0.997 0 Note. Model 1: Adjusted for demographic variables (age, sex, BMI) + variable showing significant differences between 2 groups (current smoker, prior PCI or CABG, LVEF, AMI, HbA1c, uric acid, LDL-C, SNYTAX score, no. of target lesions, no. of stent per patient, time of procedure, primary PCI, DAPT, statin, calcium antagonist, β-blocker). MACCE, major adverse cardiac and cerebrovascular events. Table 3. HRs of all-cause death, MACCE and cardiac death within 2 years of CAD patients who received PCI on off days or workdays in model 2−5
Model All-cause death MACCE Cardiac death HR (95% CI) P value Wald value HR (95% CI) P value Wald value HR (95% CI) P value Wald value Model 2 3.93 (1.73, 8.92) 0.001 10.732 1.52 (1.004, 2.29) 0.048 3.923 4.67 (1.70, 12.78) 0.003 8.977 Model 3 4.22 (1.86, 9.59) 0.001 11.79 1.52 (1.009, 2.30) 0.045 4.001 4.87 (1.77, 13.39) 0.002 9.417 Model 4 1.78 (0.73, 4.37) 0.206 1.602 1.15 (0.75, 1.76) 0.525 0.405 1.21 (0.40, 3.65) 0.737 0.113 Model 5 1.23 (0.46, 3.28) 0.674 0.177 0.92 (0.57, 1.49) 0.746 0.105 1.04 (0.32, 3.35) 0.948 0.004 Note. Model 2: Unadjusted. Model 3: Adjusted for only demographic variables (age, sex, BMI). Model 4: Adjusted for demographic variables (age, sex, BMI) + severity variables (coexisting conditions (diabetes, hypertension, hyperlipidemia, prior PCI or CABG, previous MI, current smoker, family history of CAD, CVD, COPD, PVD, LVEF), clinical presentation (AMI) and lesion characteristics (SNYTAX score, LM or TVD, LAD involved). Model 5: Adjusted for demographic variables (age, sex, BMI) + medical management variables (DAPT, β-blocker, Statin, Calcium antagonist) + invasive management variables (no. of target lesions, no. of stent per patient, primary PCI, stent type, residual SNYTAX). MACCE, major adverse cardiac and cerebrovascular events. In China, patients commonly worry about the reduced staff and less senior experienced staff in governmental hospitals during the weekends or on holidays and tend to put off the plan of seeking medical care. It was found that the worse outcomes in patients who were admitted at weekends (or off days) were partially attributable to the staff arrangement[5]. Due to this mentality, patients in pursuit of medical help on off days are probably always those with emergent diseases such as AMI and acute cerebrovascular events, and often more severe than those who see a doctor on weekdays.
An association between time of admission and short-term mortality in patients with AMI was reported. Patients with MI were more likely to die in the hospital if they were admitted on the weekend than those hospitalized on a weekday. Korean researchers found that the differences in mortality between AMI patients who were admitted on weekdays and those admitted on weekends can be explained by the different rates of medical or invasive procedures[8]. However, not all the literature supported the “weekend/holiday effect,” such as a cohort study including 62,814 AMI patients from 379 hospitals[9]. No measurable differences were found in in-hospital mortality between the regular hours and off-hours hospitalized cases in the overall AMI, STEMI, and NSTEMI cohorts. Similar observations were made across age and sex subgroups and with an alternative definition for arrival time (weekends/holidays versus weekdays). To verify the “off days effect” in the Chinese population, we conducted this research in a large all-spectrum cohort of CAD patients undergoing PCI. We found that: 1) the “off days effect” also existed in patients with CAD undergoing PCI in China. Risks of all-cause death, cardiac death, and ST in the PCI on off days group was significantly higher compared with the PCI on workdays group; 2) the “off days effect” may be explained by baseline differences including severity characteristics, medication, and invasive management. PCI on off days was not an independent predictor of mid-long-term mortality in patients with CAD undergoing PCI.
Baseline analysis showed that the PCI on off days group presented with more patients diagnosed with AMI (accounted for 84.3% in the PCI on off days group, especially those with STEMI accounted for 77.6%), more primary PCI, more complicated lesions, and less secondary prevention medication than the PCI on workdays group. The predominance of AMI may be the main reason for more primary PCI, as well as less no. of target lesions and no. of stent per patient in the PCI on off days group. Only the incriminated vessel was preferentially treated according to international guidelines during primary PCI in patients with AMI and multi-vessel disease. Meanwhile, the cases in the PCI on off days group were less prescribed aspirin, statin, and β-blocker than those in the PCI on workdays group, which may explain the increased mortality before multivariate adjustment to some extent. Particularly, less dual antiplatelet therapy may be related to increased ST risk before multivariate adjustment in the PCI on the off days group.
In studies investigating the “off-time effect” in patients with STEMI, patients with STEMI presenting during off-hours were less likely to receive PCI within 90 minutes and had a longer door-to-balloon time compared with cases with an onset during regular hours[1,10]. In addition, it was suggested that future studies should figure out the variation in the quality of medical care by the time of the day, such as the number of staff, staff expertise, and other workflow defects during off-hours in the care system. In other words, they considered that the “off-time effect” was essentially a manifestation of hospital management deficiency. More reasonable staff arrangement, especially team scheduling of primary PCI, standardized management of perioperative workflow, may directly influence the quality of medical care and prognosis of patients with STEMI.
This study revealed similar results to those of previous reports but had its own characteristics. Our data was collected from the largest cardiovascular center in Northern China, with a large quantity of more than ten thousand PCI cases every year since 2013. Operators responsible for primary PCI during off time are experienced interventional cardiologists. The workflow of primary PCI has been matured in our center, including emergency reception, handling, chief resident coordination, PCI team in place, and subsequent treatment in the coronary care unit after the procedures. The staffing status of our catheter room includes an operator and several assistants (always one or two trainee doctors) on duty, a nurse, and several chores aunts on duty. Medical staffing in the coronary care unit on the weekend include a chief resident and two senior residents on duty. Moreover, most hospitalized patients in this study were nonlocal residents with complicated coexisting conditions or coronary lesions. Therefore, the results drawn out in this study only presents the situation in our center, and cannot represent other tertiary centers, notably cannot be extrapolated to local primary hospitals.
Admittedly, several limitations must be taken into consideration. Firstly, residual confounders inevitably existed in this observational study, such as compliance of secondary prevention drugs during follow-up, hemodynamics status, and seniority of operators. Secondly, there is a lack of clock data. The study of PCI time cannot go deep into the circadian rhythm. Nevertheless, it was the first study to analyze the effect of PCI time on long-term outcomes in patients with all-spectrum CAD undergoing PCI in China. Future research should address the root causes for such care gaps and explore the clinical impact of implementing quality initiatives to improve these management shortfalls. Efforts to improve systems of care should ensure that comparable outcomes are achieved for patients regardless of the time of day or day of the week that the invasive procedures take place.
Long-term mortality of patients who underwent PCI on holidays or weekends was higher compared with those undergoing PCI on workdays. However, PCI on holidays or weekends was not independently associated with the 2-year mortality. The coexisting conditions, complexity of lesions, predominance of AMI, application discrepancy of the secondary prevention medication, and invasive management were all contributing to the worse outcomes in patients with CAD who received PCI on off days.
-
Ethics Ethical approvals were obtained from the Fuwai Hospital Research Ethics Committees (No. 2013-449).
Consent to publish Not applicable
Availability of data and materials The data used to support the findings of this study are available from the corresponding author upon request.
Competing interests The authors declare that they have no competing interests.
Authors' Contributions RL contributed to all aspects of this study, including the study conception and design, data acquisition, statistical analysis and interpretation, and drafting and revising the report. LJG, CZ, SDJ, SBQ, YJY, and RLG contributed to data acquisition. BX and JQY contributed to the initial study conception and design, data interpretation, and critical revision of the report. All authors have approved the final article.
Acknowledgements We are grateful to the staff in the Department of Cardiology and Catheterization Laboratory, Fu Wai Hospital, for their research contributions.
doi: 10.3967/bes2021.051
Does Percutaneous Coronary Intervention on Off Days have an Effect on Long-term Prognosis in Patients with Coronary Artery Disease in China?
-
-
Table 1. The baselines clinical, angiographic, procedural characteristics, medication situation and 2-year outcomes between the two groups
Variables All (n = 10,724) PCI on off days (n = 134) PCI on workdays (n = 10,590) P value Demographic characteristics Male gender, n (%) 8,272 (77.1) 105 (78.4) 8,167 (77.1) 0.734 Age, years 58.4 ± 10.3 58.2 ± 11.7 58.4 ± 10.3 0.900 BMI, kg/m2 25.9 ± 3.2 26.7 ± 3.6 25.9 ± 3.2 0.014 Coexisting conditions, n (%) Hypertension 6,906 (64.4) 83 (61.9) 6,823 (64.4) 0.550 DM 3,238 (30.2) 41 (30.6) 3,197 (30.2) 0.919 Hyperlipidemia 7,211 (67.2) 91 (67.9) 7,120 (67.2) 0.868 Previous MI 2,061 (19.2) 24 (17.9) 2,037 (19.2) 0.699 Prior PCI or CABG 2,808 (26.2) 83 (61.9) 2,725 (25.7) < 0.001 Current smoker 6,123 (57.1) 90 (67.2) 6,033 (57.0) 0.018 Family history of CAD 2,651 (24.7) 31 (23.1) 2,620 (24.8) 0.666 CVD 1,150 (10.7) 19 (14.2) 1,131 (10.7) 0.193 PVD 288 (2.7) 5 (3.7) 283 (2.7) 0.451 COPD 247 (2.3) 0 (0.0) 247 (2.3) 0.074 LVEF (%) 62.8 ± 7.4 54.9 ± 8.3 62.9 ± 7.2 < 0.001 Clinical presentation, n (%) Asymptomatic ischemia 869 (8.1) 3 (2.2) 866 (8.2) < 0.001 Stable angina 3,424 (31.9) 5 (3.7) 3,419 (32.3) < 0.001 Unstable angina pectoris 4,509 (42.0) 13 (9.7) 4,496 (42.5) < 0.001 AMI 1,922 (17.9) 113 (84.3) 1,809 (17.1) < 0.001 STEMI 1,447 (13.5) 104 (77.6) 1,343 (12.7) < 0.001 NSTEMI 475 (4.4) 9 (6.7) 466 (4.4) 0.195 Laboratory examination eGFR before PCI, mL·min−1·1.73 m−2 91.3 ± 15.1 89.4 ± 19.4 91.3 ± 15.1 0.256 HGB before PCI, g/L 141.0 ± 15.8 141.0 ± 16.5 141.0 ± 15.8 0.985 PLT before PCI, 109/L 203.6 ± 54.4 199.2 ± 77.0 203.6 ± 54.1 0.348 Uric acid, µmol/L 341.6 ± 84.7 324.5 ± 90.5 341.8 ± 84.6 0.018 HbA1c, % 6.6 ± 1.2 7.0 ± 1.7 6.6 ± 1.2 0.008 LDL-C, mmol/L 2.50 ± 0.90 2.74 ± 0.83 2.50 ± 0.90 0.003 ESR, mm/h 10.8 ± 11.3 10.8 ± 11.0 10.8 ± 11.3 0.988 Angiographic and procedural characteristics SNYTAX score 11.7 ± 8.1 14.8 ± 8.6 11.7 ± 8.1 < 0.001 Residual SNYTAX 3.4 ± 5.7 4.4 ± 6.9 3.4 ± 5.7 0.100 LM or TVD, n (%) 457 (4.3) 2 (1.5) 455 (4.3) 0.110 LAD involved, n (%) 9,702 (90.5%) 124 (92.5) 9,578 (90.4) 0.412 No. of target lesions 1.40 ± 0.66 1.29 ± 0.62 1.41 ± 0.66 0.037 No. of stent per patient 1.80 ± 1.11 1.52 ± 1.05 1.81 ± 1.11 0.002 Time of procedure, min 36.7 ± 31.5 46.7 ± 41.6 36.5 ± 31.4 0.006 Primary PCI, n (%) 323 (3.0) 108 (80.6) 215 (2.0) < 0.001 Procedure and stent type, n (%) 0.079 PTCA 237 (2.2) 7 (5.2) 230 (2.2) BMS 64 (0.6) 2 (1.5) 62 (0.6) First-generation durable polymer DES 597 (5.6) 7 (5.2) 590 (5.6) Second-generation durable polymer DES 6,094 (56.8) 80 (59.7) 6,014 (56.8) Domestic biodegradable polymer DES 1,572 (14.7) 13 (9.7) 1,559 (14.7) Mixed implantation of DES 1,692 (15.8) 19 (14.2) 1,673 (15.8) Others (Janus, Yinyi) 167 (1.6) 4 (3.0) 163 (1.5) Procedure unsuccess 301 (2.8) 2 (1.5) 299 (2.8) Medication, n (%) Aspirin 10,585 (98.7) 125 (93.3) 10,460 (98.8) < 0.001 Clopidogrel 10,701 (99.8) 134 (100.0) 10,567 (99.8) 0.589 Ticagrelor 19 (0.2) 0 (0.0) 19 (0.2) 0.624 DAPT 10,583 (98.7) 125 (93.3) 10,458 (98.8) < 0.001 Statin 10,285 (95.9) 119 (88.8) 10,166 (96.0) < 0.001 Calcium antagonist 5,216 (48.6) 50 (37.3) 5,166 (48.8) 0.008 β-blocker 9,673 (90.2) 113 (84.3) 9,560 (90.3) 0.021 2-year outcomes, n (%) All-cause death 131 (1.2) 6 (4.5) 125 (1.2) 0.001 MACCE 1,295 (12.1) 23 (17.2) 1,272 (12.0) 0.069 Cardiac death 74 (0.7) 4 (3.0) 70 (0.7) 0.001 Myocardial infarction 212 (2.0) 4 (3.0) 208 (2.0) 0.399 Revascularization 923 (8.6) 14 (10.4) 909 (8.6) 0.445 Stent thrombosis 91 (0.8) 4 (3.0) 87 (0.8) 0.007 Stroke 145 (1.4) 2 (1.5) 143 (1.4) 0.887 Bleeding 702 (6.5) 6 (4.5) 696 (6.6) 0.330 Note. AMI, acute myocardial infarction; BMI, body mass index; BMS, bare metal stent; CAD, coronary artery disease; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CVD, cerebral vascular disease; DES, drug-eluting stent; DM, diabetes mellitus; DAPT, dual anti-platelet therapy; eGFR, estimated glomerular filtration rate; ESR, erythrocyte sedimentation rate; HGB, hemoglobin; HbA1c, hemoglobin A1c; LAD, left anterior descending artery; LM, left main; LDL-C, low density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non-ST-segment elevated myocardial infarction; PCI, percutaneous coronary intervention; PLT, platelet; PTCA, percutaneous transluminal coronary angioplasty; PVD, peripheral vascular disease; STEMI, ST-segment elevated myocardial infarction; SYNTAX, Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery; TC, total cholesterol; TVD, three-vessel disease. Data are expressed as mean ± standard deviation; or counts (percentage). Table 2. HRs of events within 2 years of CAD patients who received PCI on off days or workdays in model 1
Item HR (95% CI) P value Wald value All-cause death 0.91 (0.34, 2.43) 0.854 0.034 MACCE 0.86 (0.53, 1.39) 0.539 0.378 Cardiac death 0.77 (0.24, 2.48) 0.658 0.195 Myocardial infarction 0.64 (0.21, 1.94) 0.431 0.619 Revascularization 0.91 (0.49, 1.68) 0.765 0.089 Stent thrombosis 1.36 (0.39, 4.71) 0.629 0.233 Stroke 0.50 (0.11, 2.28) 0.367 0.814 Bleeding 1.00 (0.40, 2.49) 0.997 0 Note. Model 1: Adjusted for demographic variables (age, sex, BMI) + variable showing significant differences between 2 groups (current smoker, prior PCI or CABG, LVEF, AMI, HbA1c, uric acid, LDL-C, SNYTAX score, no. of target lesions, no. of stent per patient, time of procedure, primary PCI, DAPT, statin, calcium antagonist, β-blocker). MACCE, major adverse cardiac and cerebrovascular events. Table 3. HRs of all-cause death, MACCE and cardiac death within 2 years of CAD patients who received PCI on off days or workdays in model 2−5
Model All-cause death MACCE Cardiac death HR (95% CI) P value Wald value HR (95% CI) P value Wald value HR (95% CI) P value Wald value Model 2 3.93 (1.73, 8.92) 0.001 10.732 1.52 (1.004, 2.29) 0.048 3.923 4.67 (1.70, 12.78) 0.003 8.977 Model 3 4.22 (1.86, 9.59) 0.001 11.79 1.52 (1.009, 2.30) 0.045 4.001 4.87 (1.77, 13.39) 0.002 9.417 Model 4 1.78 (0.73, 4.37) 0.206 1.602 1.15 (0.75, 1.76) 0.525 0.405 1.21 (0.40, 3.65) 0.737 0.113 Model 5 1.23 (0.46, 3.28) 0.674 0.177 0.92 (0.57, 1.49) 0.746 0.105 1.04 (0.32, 3.35) 0.948 0.004 Note. Model 2: Unadjusted. Model 3: Adjusted for only demographic variables (age, sex, BMI). Model 4: Adjusted for demographic variables (age, sex, BMI) + severity variables (coexisting conditions (diabetes, hypertension, hyperlipidemia, prior PCI or CABG, previous MI, current smoker, family history of CAD, CVD, COPD, PVD, LVEF), clinical presentation (AMI) and lesion characteristics (SNYTAX score, LM or TVD, LAD involved). Model 5: Adjusted for demographic variables (age, sex, BMI) + medical management variables (DAPT, β-blocker, Statin, Calcium antagonist) + invasive management variables (no. of target lesions, no. of stent per patient, primary PCI, stent type, residual SNYTAX). MACCE, major adverse cardiac and cerebrovascular events. -
[1] Sorita A, Ahmed A, Starr SR, et al. Off-hour presentation and outcomes in patients with acute myocardial infarction: systematic review and meta-analysis. BMJ, 2014; 348, f7393. doi: 10.1136/bmj.f7393 [2] Kostis WJ, Demissie K, Marcella SW, et al. Weekend versus weekday admission and mortality from myocardial infarction. N Engl J Med, 2017; 356, 1099−109. [3] Handel AE, Patel SV, Skingsley A, et al. Weekend admissions as an independent predictor of mortality: an analysis of Scottish hospital admissions. BMJ Open, 2012; 2, e001789. doi: 10.1136/bmjopen-2012-001789 [4] Ju MJ, Tu GW, Han Y, et al. Effect of admission time on mortality in an intensive care unit in mainland china: a propensity score matching analysis. Crit Care, 2013; 17, R230. doi: 10.1186/cc13053 [5] Aylin P, Alexandrescu R, Jen MH, et al. Day of week of procedure and 30 day mortality for elective surgery: retrospective analysis of hospital episode statistics. BMJ, 2013; 346, f2424. [6] Attenello FJ, Wen T, Cen SY, et al. Incidence of “never events” among weekend admissions versus weekday admissions to us hospitals: national analysis. BMJ, 2015; 350, h1460. doi: 10.1136/bmj.h1460 [7] Liu R, Gao Z, Gao LJ, et al. Risk or beneficial factors associated with unplanned revascularization risk following percutaneous coronary intervention: a large single-center data. Biomed Environ Sci, 2020; 33, 431−43. [8] Hong JS, Kang HC, Lee SH. Comparison of case fatality rates for acute myocardial infarction in weekday vs weekend admissions in South Korea. Circ J, 2010; 74, 496−502. doi: 10.1253/circj.CJ-09-0678 [9] Jneid H, Fonarow GC, Cannon CP, et al. Impact of time of presentation on the care and outcomes of acute myocardial infarction. Circulation, 2008; 117, 2502−9. doi: 10.1161/CIRCULATIONAHA.107.752113 [10] Casella G, Ottani F, Ortolani P, et al. Off-hour primary percutaneous coronary angioplasty does not affect outcome of patients with ST-Segment elevation acute myocardial infarction treated within a regional network for reperfusion: The REAL (Registro Regionale Angioplastiche dell'Emilia-Romagna) registry. JACC Cardiovasc Interv, 2011; 4, 270−8. doi: 10.1016/j.jcin.2010.11.012 -




 下载:
下载:




 Quick Links
Quick Links