-
Terahertz (THz) waves, also known as T-rays, encompass frequencies ranging from 0.1 to 10 THz and possess unique properties that render them applicable in various biomedical domains, particularly in neurobiology[1]. Synaptic transmission, the process through which signals propagate between neurons at synapses, is pivotal for brain function and information processing. Disruptions in synaptic transmission can contribute to neurological disorders such as Alzheimer’s disease, Parkinson’s disease, and epilepsy. Hence, comprehending the impact of THz waves on synaptic transmission holds significance for fundamental neuroscientific research and potential therapeutic interventions. This study aims to explore a novel neuromodulation approach for noninvasive regulation of neuronal function.
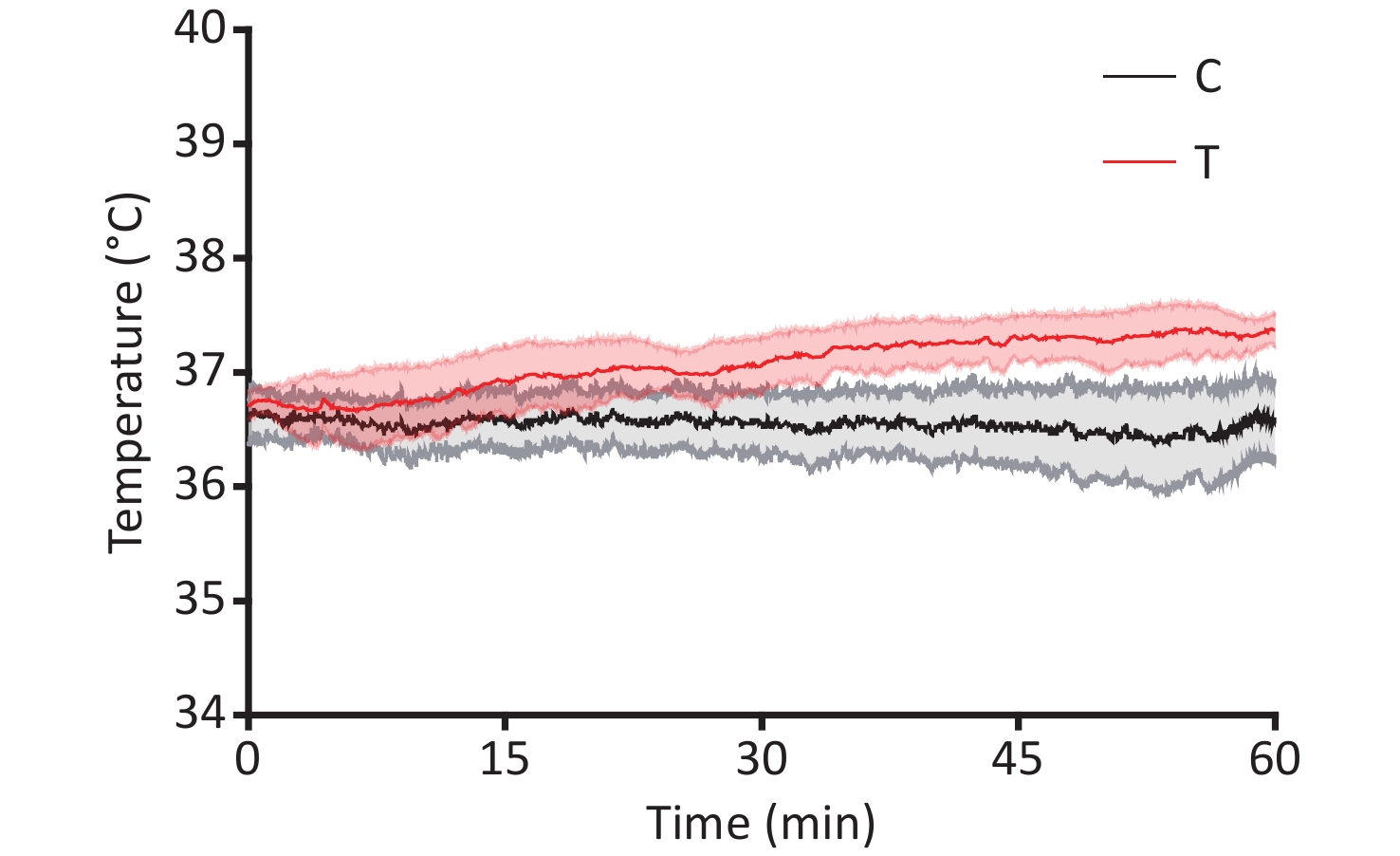
The cultivation of male Wistar rat primary hippocampal neurons and the THz wave exposure systems have been extensively described in the literature[2]. Primary hippocampal neurons were subjected to THz waves with a frequency of 2 THz and a power of 3.5 μW, generated by a QS1-260-HP source (frequency range of 0.114–2.349 THz), for 60 minutes. On the 7th day of culture, primary hippocampal neurons were categorized into control (C) and radiation (R) groups based on whether they were exposed to THz waves. The R group underwent a 60-minute exposure to 2 THz waves, while the C group remained unexposed. Exposure to 2 THz waves for 60 minutes led to a temperature elevation of approximately 0.839 °C. The temperature rise in the cell culture medium during THz wave exposure was less than 1 °C (Supplementary Figure S1, available in www.besjournal.com), suggesting that nonthermal effects were predominantly documented during THz wave exposure. The arithmetic means of all results are presented in Supplementary Table S1 (available in www.besjournal.com).
Table S1. Arithmetic mean of experimental results ($ \overline{X}\pm SEM) $
Experimental project C R Temperature 36.59 ± 0.34 37.37 ± 0.15 Western Blot VAMP2 1.00 ± 0.05 1.10 ± 0.08 SNAP 1.00 ± 0.04 1.34 ± 0.39 rSec6 1.00 ± 0.07 1.16 ± 0.01*** Synaptophysin 1.00 ± 0.02 1.17 ± 0.12 GluN1 1.00 ± 0.10 0.92 ± 0.12 GluN2A 1.00 ± 0.25 1.05 ± 0.24 GluN2B 1.00 ± 0.09 1.42 ± 0.12* GluA1 1.00 ± 0.03 1.17 ± 0.06* PSD95 1.00 ± 0.07 1.08 ± 0.03* CaMKII 1.00 ± 0.17 1.03 ± 0.04 nNOS 1.00 ± 0.06 1.25 ± 0.04* CREB 1.00 ± 0.09 1.26 ± 0.04** Neurotransmitter Asp 1.00 ± 0.20 0.74 ± 0.34 Glu 1.00 ± 0.05 1.15 ± 0.03* Gly 1.00 ± 0.16 0.59 ± 0.15* Ca2+ concentration Cytoplasm 1.00 ± 0.03 0.86 ± 0.03* Endoplasmic reticulum 1.00 ± 0.09 1.52 ± 0.18* Mitochondria 1.00 ± 0.13 1.73 ± 0.07** Voltage-gated calcium channel activity −10 mV −9.90 ± 1.16 −13.29 ± 1.22* 0 mV −14.26 ± 1.89 −18.49 ± 1.91* Note. Compared to the C group, *P < 0.05, **P < 0.01, ***P < 0.001. Total protein was extracted from primary hippocampal neurons at 6 hours after exposure. Western blotting was employed to assess the protein content of primary hippocampal neurons, following previously established protocols[3]. Western blotting results for presynaptic signaling molecules in primary hippocampal neurons are depicted in Figure 1A. In comparison to group C, group R exhibited elevated levels of rSec6 (P < 0.05). However, the levels of VAMP2, SNAP, and Synaptophysin did not exhibit significant changes (P > 0.05, Figure 1B). Western blot results for the glutamate receptor subunits are illustrated in Figure 1C, revealing significantly increased levels of GluN2B and GluA1 (P < 0.05) in the R group (Figure 1D). The Western blot results for postsynaptic signaling molecules are presented in Figure 1E. Comparative analysis with the C group revealed a significant increase in PSD95, nNOS, and CREB levels in the R group (P < 0.05, P < 0.05, P < 0.01, Figure 1F). Studies on synaptic plasticity are pivotal for understanding learning, memory, development, and neurological disorders[4]. Neuronal synaptic plasticity is influenced by various processes, including presynaptic neurotransmitter release, neurotransmitter-receptor binding, and postsynaptic signal transduction. These processes collectively determine the formation and maintenance of synaptic plasticity. Following exposure to THz waves, the expression of the presynaptic vesicle-related molecule rSEC6 increased. Moreover, the levels of the GluN2B and GluA1 subunits of glutamate receptors increased, alongside elevated levels of the postsynaptic signaling molecules PSD95, nNOS, and CREB. Therefore, THz waves enhance synaptic plasticity by promoting presynaptic vesicle synthesis and release, strengthening postsynaptic signal transmission, and increasing the number of postsynaptic glutamate receptor subunits.
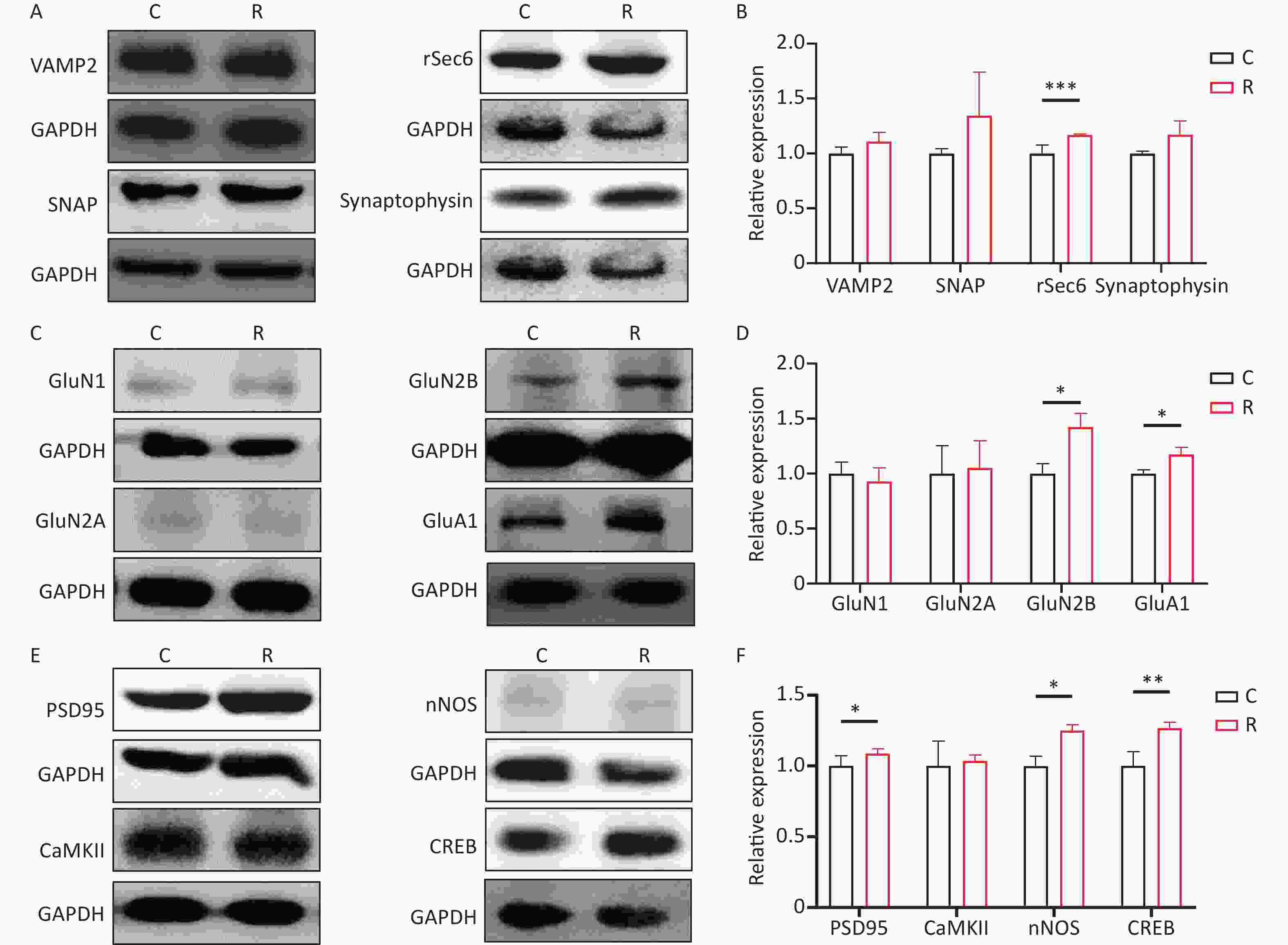
Figure 1. Expression of Synaptic plasticity-related molecules in primary hippocampal neurons after 2 THz exposure. (A) The Western blot of presynaptic signaling molecules after exposure to 2 THz. (B) Western blot quantitative analysis of presynaptic signaling molecules. (C) The Western blot of glutamate receptor subunits after exposure to 2 THz. (D) Western blot quantitative analysis of glutamate receptor subunits. (E) The Western blot of postsynaptic signaling molecules after exposure to 2 THz. (F) Western blot quantitative analysis of postsynaptic signaling molecules. THz, terahertz; C, control group; R, radiation group. Compared to the C group, *P < 0.05, **P < 0.01, ***P < 0.001.
At 6 hours after THz exposure, 300 µL of primary hippocampal neuronal medium was aspirated and assayed for aspartate (Asp), glutamate (Glu), and glycine (Gly) using High Performance Liquid Chromatography (HPLC). Following 2 THz exposure, neurotransmitter levels in the cell medium were assessed via HPLC. The results depicted in Figure 2A reveal elevated levels of glutamate in the R group (P < 0.05), alongside a decrease in glycine levels (P < 0.05). Consequently, THz waves appear to enhance the release of the excitatory neurotransmitter glutamate while concurrently reducing the release of the inhibitory neurotransmitter glycine. Neurotransmitters serve as crucial molecules in the nervous system, facilitating signal transmission and regulating communication between neurons. They can be classified into excitatory and inhibitory neurotransmitters, which exert excitatory and inhibitory effects on postsynaptic membranes, respectively[5]. After exposure to THz waves, there was a noticeable increase in excitatory neurotransmitter glutamate levels, coupled with a decrease in inhibitory neurotransmitter aspartate levels in the primary hippocampal neuron culture medium. This suggests that THz waves may influence the function of primary hippocampal neurons by modulating the levels of excitatory and inhibitory neurotransmitters.
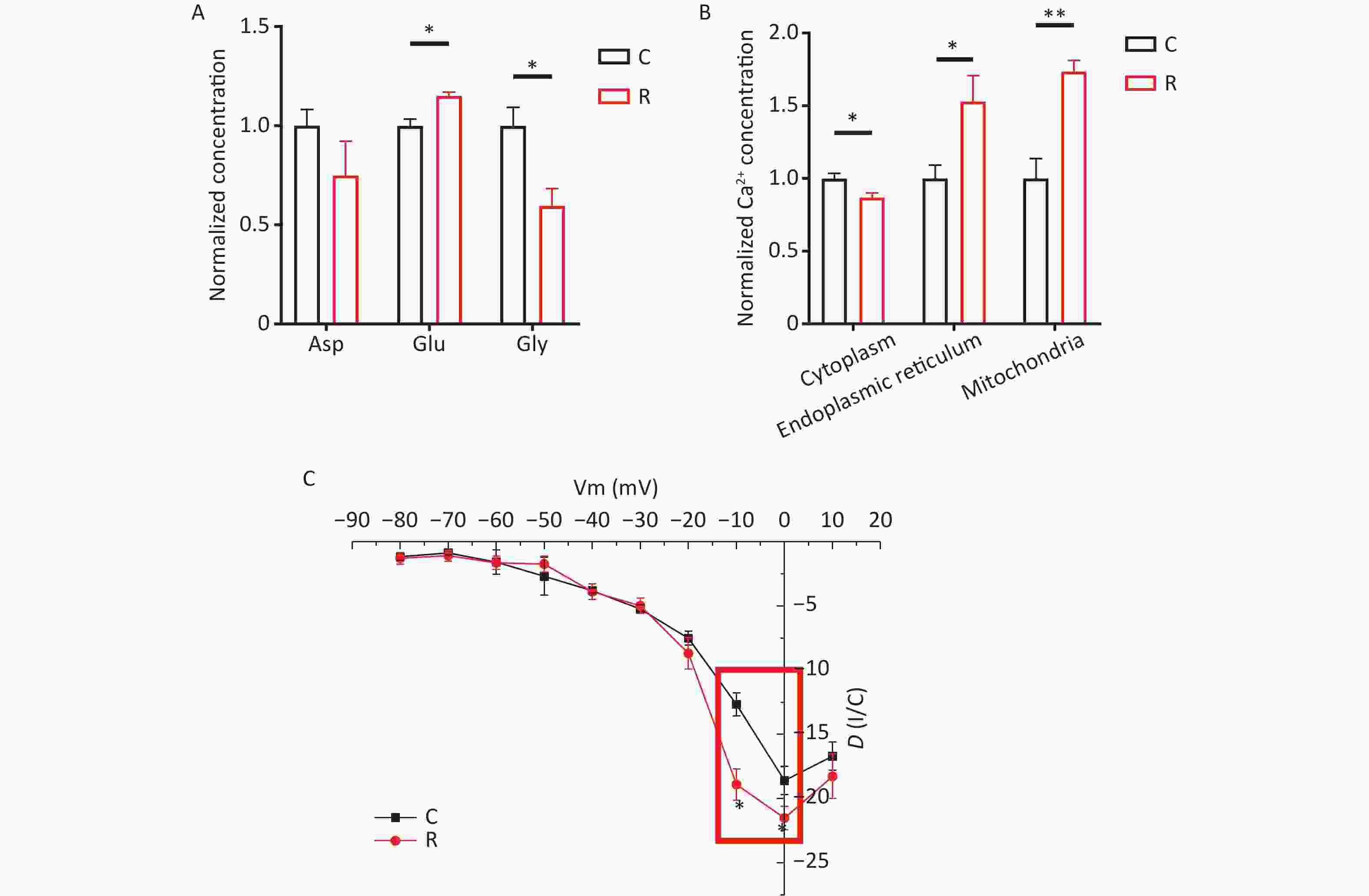
Figure 2. Changes in neurotransmitter, calcium ion, and voltage-gated calcium channel activity in primary hippocampal neurons following exposure to 2 THz. (A) Neurotransmitter changes after exposure to 2 THz. (B) Calcium ion alterations after exposure to 2 THz. (C) Changes in voltage-gated calcium channel activity after exposure to 2 THz. THz, terahertz; C, control group; R, radiation group. Compared to the C group, *P < 0.05, **P < 0.01.
To assess the Ca2+ content in various subcellular structures of primary hippocampal neurons, we measured the relative intensity of cytoplasmic, endoplasmic reticulum, and mitochondrial Ca2+ levels using a GENMED Calcium Ion Fluorescence Quantification Kit (GENMED, USA). As show depicted in Figure 2B, compared to the C group, the cytoplasmic Ca2+ concentration significantly decreased in the R group (P < 0.05). Conversely, the concentrations of Ca2+ in the endoplasmic reticulum (P < 0.05) and mitochondria (P < 0.01) increased, indicating that THz waves prompted the transfer of Ca2+ from the cytoplasm to these organelles. In neuronal cells, Ca²⁺ concentration plays a pivotal role in maintaining normal neural signal transmission and cellular functions[6]. The concentration of Ca2+ in neuronal cytoplasm is closely associated with neuronal excitability and synaptic transmission. The endoplasmic reticulum is responsible for protein synthesis and folding within cells. Alterations in Ca²⁺ concentration could influence the folding status of proteins in the endoplasmic reticulum, thereby impacting intracellular protein synthesis. Mitochondria serve as energy production centers within cells, and their Ca2+ concentration can affect mitochondrial respiratory chain activity, thereby influencing cellular energy supply[7,8]. In this study, exposure to THz waves significantly increased the concentrations of Ca2+ in the endoplasmic reticulum and mitochondria of primary hippocampal neurons, while cytoplasmic Ca²⁺ concentrations exhibited a decreasing trend. Therefore, THz waves can enhance cellular energy metabolism by facilitating the transfer of Ca2+ from the cytoplasm to the endoplasmic reticulum and mitochondria of primary hippocampal neurons.
Primary hippocampal neuronal calcium currents were recorded using whole-cell patch-clamp techniques. The I-V curves of voltage-gated calcium channels in these neurons are presented in Figure 2C. Compared to the C group, the current density of voltage-gated calcium channels showed significantly higher absolute values at membrane potentials of -10 and 0 mV in the R group (P < 0.05). Neuronal electrical activity is essential for information transmission in the nervous system and plays a crucial role in physiological processes such as perception, learning, memory, and motor control. Research suggests that THz waves may influence neuronal electrical activity by altering the electric field and membrane potential of neurons, leading to changes in neuronal electrophysiological properties, including membrane potential and firing frequency of action potentials. Some researchers found that exposing rat hippocampal brain slices to THz waves with a frequency of 0.138 THz and a power of 2 mW increased the slope of excitatory postsynaptic potentials during exposure, enhancing synaptic plasticity in the Schaeffer-CA1 region. They also observed that exposure to 0.138 THz promoted neurite outgrowth and increased dendritic spine density[9]. Mathematical models simulating the dynamics of K+ and Na+ channels have shown that carbonyl groups (-C=O) in the functional domain of K+ channels can resonate and absorb photon energy from THz waves, thereby increasing K+ permeability[10]. Calcium currents in neurons are critical for the generation and propagation of action potentials, playing a primary role in action potential generation, postsynaptic signal transduction, and long-term potentiation. In this study, whole-cell patch-clamp recordings were used to record calcium currents. The results indicated that THz waves enhanced the activity of voltage-gated calcium channels, as evidenced by an absolute increase in current density, suggesting increased excitability of primary hippocampal neurons. These findings are consistent with previous studies reporting the impact of THz waves on neuronal electrical activity. However, the regulatory effects and mechanisms of THz waves on neuronal electrical activity under different conditions may vary and require further exploration.
In summary, primary hippocampal neurons exhibited enhanced synaptic transmission following exposure, as depicted in Figure 3. At the synaptic level, THz waves enhance presynaptic vesicular transport, leading to increased release of excitatory neurotransmitters and the overexpression of glutamate receptor subunits and proteins involved in postsynaptic signal transduction pathways. These effects contribute to the enhancement of synaptic plasticity in primary hippocampal neurons. At the neuronal level, THz waves augment protein synthesis and metabolic activity in primary hippocampal neurons by increasing the levels of calcium ions in the endoplasmic reticulum and mitochondria, thereby boosting neuronal excitability. Additionally, exposure to THz waves resulted in elevated calcium currents. The observed electrophysiological enhancements align with changes in synaptic plasticity, indicating a positive modulation of synaptic transmission in primary hippocampal neurons by THz waves.
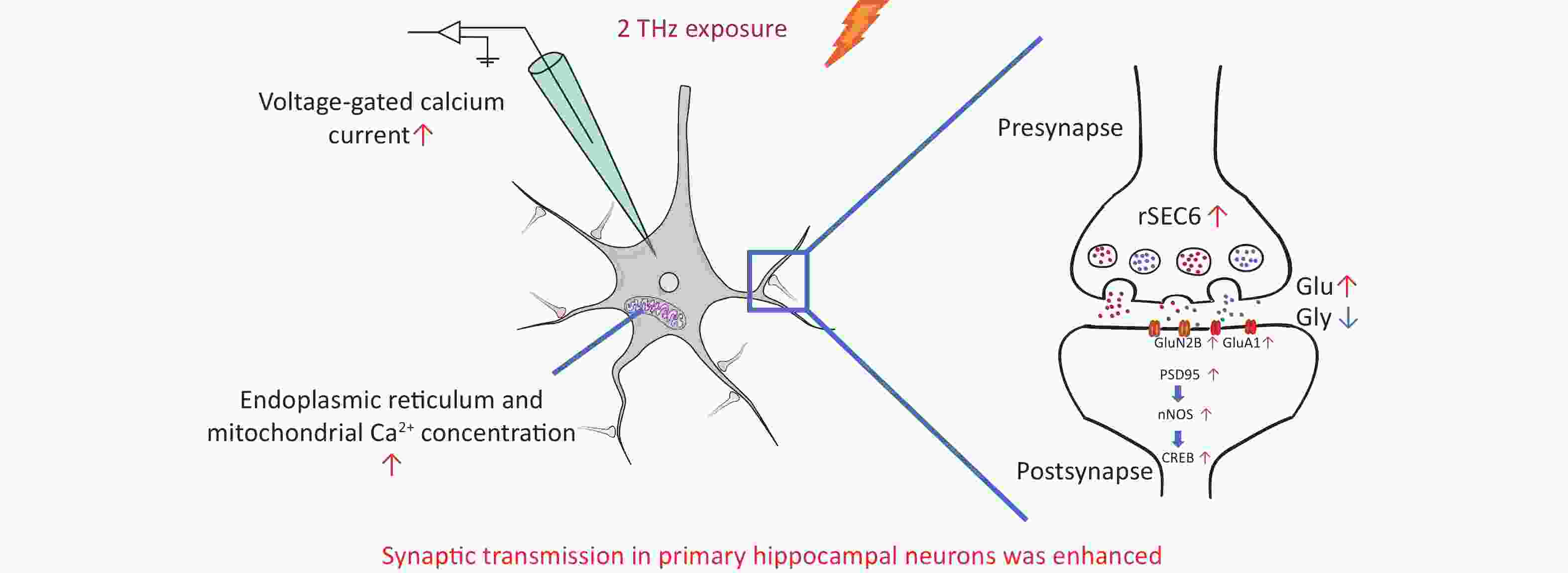
Figure 3. Schematic representation of THz waves enhancing synaptic transmission in primary hippocampal neurons. THz, terahertz; Glu, glutamate; Gly, andglycine.
This study offers new insights into the mechanisms by which THz waves affect the nervous system, potentially opening new treatment avenues for neurodegenerative diseases like Alzheimer’s and Parkinson’s. However, it is crucial to highlight that while the findings suggesting the potential of THz waves in this context, more thorough research is needed. This should include investigating potential risks and safety concerns before considering practical applications.
Author Contributions Hui Wang, Li Zhao and Ruiyun Peng designed this work. Lequan Song, Zhiwei He and Junmiao Pan performed the experiments and analyzed the data. Lequan Song wrote the paper. Ji Dong, Haoyu Wang, Jing Zhang, Binwei Yao and Xinping Xu participated in its design and coordination and helped to draft the manuscript. All authors participated in critically revising and approving the final manuscript.
doi: 10.3967/bes2024.099
Synaptic Transmission of Primary Hippocampal Neurons was Enhanced after Terahertz Waves Exposure
-
&These authors contributed equally to this work.
注释: -
Figure 1. Expression of Synaptic plasticity-related molecules in primary hippocampal neurons after 2 THz exposure. (A) The Western blot of presynaptic signaling molecules after exposure to 2 THz. (B) Western blot quantitative analysis of presynaptic signaling molecules. (C) The Western blot of glutamate receptor subunits after exposure to 2 THz. (D) Western blot quantitative analysis of glutamate receptor subunits. (E) The Western blot of postsynaptic signaling molecules after exposure to 2 THz. (F) Western blot quantitative analysis of postsynaptic signaling molecules. THz, terahertz; C, control group; R, radiation group. Compared to the C group, *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 2. Changes in neurotransmitter, calcium ion, and voltage-gated calcium channel activity in primary hippocampal neurons following exposure to 2 THz. (A) Neurotransmitter changes after exposure to 2 THz. (B) Calcium ion alterations after exposure to 2 THz. (C) Changes in voltage-gated calcium channel activity after exposure to 2 THz. THz, terahertz; C, control group; R, radiation group. Compared to the C group, *P < 0.05, **P < 0.01.
S1. Arithmetic mean of experimental results ($ \overline{X}\pm SEM) $
Experimental project C R Temperature 36.59 ± 0.34 37.37 ± 0.15 Western Blot VAMP2 1.00 ± 0.05 1.10 ± 0.08 SNAP 1.00 ± 0.04 1.34 ± 0.39 rSec6 1.00 ± 0.07 1.16 ± 0.01*** Synaptophysin 1.00 ± 0.02 1.17 ± 0.12 GluN1 1.00 ± 0.10 0.92 ± 0.12 GluN2A 1.00 ± 0.25 1.05 ± 0.24 GluN2B 1.00 ± 0.09 1.42 ± 0.12* GluA1 1.00 ± 0.03 1.17 ± 0.06* PSD95 1.00 ± 0.07 1.08 ± 0.03* CaMKII 1.00 ± 0.17 1.03 ± 0.04 nNOS 1.00 ± 0.06 1.25 ± 0.04* CREB 1.00 ± 0.09 1.26 ± 0.04** Neurotransmitter Asp 1.00 ± 0.20 0.74 ± 0.34 Glu 1.00 ± 0.05 1.15 ± 0.03* Gly 1.00 ± 0.16 0.59 ± 0.15* Ca2+ concentration Cytoplasm 1.00 ± 0.03 0.86 ± 0.03* Endoplasmic reticulum 1.00 ± 0.09 1.52 ± 0.18* Mitochondria 1.00 ± 0.13 1.73 ± 0.07** Voltage-gated calcium channel activity −10 mV −9.90 ± 1.16 −13.29 ± 1.22* 0 mV −14.26 ± 1.89 −18.49 ± 1.91* Note. Compared to the C group, *P < 0.05, **P < 0.01, ***P < 0.001. -
[1] Ma SQ, Li ZW, Gong SX, et al. The laws and effects of terahertz wave interactions with neurons. Front Bioeng Biotechnol, 2023; 11, 1147684. doi: 10.3389/fbioe.2023.1147684 [2] Tan SZ, Tan PC, Luo LQ, et al. Exposure effects of terahertz waves on primary neurons and neuron-like cells under nonthermal conditions. Biomed Environ Sci, 2019; 32, 739−54. [3] Wang H, Tan SZ, Dong J, et al. iTRAQ quantitatively proteomic analysis of the hippocampus in a rat model of accumulative microwave-induced cognitive impairment. Environ Sci Pollut Res, 2019; 26, 17248−60. doi: 10.1007/s11356-019-04873-0 [4] Middei S, Ammassari-Teule M, Marie H. Synaptic plasticity under learning challenge. Neurobiol Learn Mem, 2014; 115, 108−15. doi: 10.1016/j.nlm.2014.08.001 [5] Hanada T. Ionotropic glutamate receptors in epilepsy: a review focusing on AMPA and NMDA receptors. Biomolecules, 2020; 10, 464. doi: 10.3390/biom10030464 [6] Glaser T, Castillo ARG, Oliveira Á, et al. Intracellular calcium measurements for functional characterization of neuronal phenotypes. Methods Mol Biol, 2016; 1341, 245−55. [7] Ruiz-Fernández AR, Campos L, Gutierrez-Maldonado SE, et al. Nanosecond pulsed electric field (nsPEF): opening the biotechnological pandora's box. Int J Mol Sci, 2022; 23, 6158. doi: 10.3390/ijms23116158 [8] Marchi S, Patergnani S, Missiroli S, et al. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium, 2018; 69, 62−72. doi: 10.1016/j.ceca.2017.05.003 [9] Ma SQ, Li ZW, Gong SX, et al. High frequency electromagnetic radiation stimulates neuronal growth and hippocampal synaptic transmission. Brain Sci, 2023; 13, 686. doi: 10.3390/brainsci13040686 [10] Liu X, Qiao Z, Chai YM, et al. Nonthermal and reversible control of neuronal signaling and behavior by midinfrared stimulation. Proc Natl Acad Sci USA, 2021; 118, e2015685118. doi: 10.1073/pnas.2015685118 -
 24104+Supplementary Materials.pdf
24104+Supplementary Materials.pdf

-




 下载:
下载:





 Quick Links
Quick Links