-
Rabies is a zoonotic disease caused by the rabies virus (RABV). Rabies kills approximately 59,000 people worldwide each year[1]. China has the second-highest number of rabies cases worldwide. Although rabies can be prevented by vaccination, the cost worldwide is relatively high: approximately USD 8.6 billion per year. The appearance of clinical symptoms is followed by death in almost 100% of cases[2]. Ninety-nine percent of human rabies cases are caused by canine transmission.
Rabies is a vaccine-preventable disease, and vaccinating dogs is the most cost-effective means to prevent human rabies; nevertheless, rabies prevention in China is based on human post-exposure prophylaxis (PEP), with rabies vaccination being one of its core aspects. The post-exposure immunization schedules specified in the Technical Guidelines for Human Rabies Prevention and Control (2016 version) are the Essen schedule (“1-1-1-1-1”; 1 dose of vaccine on days 0, 3, 7, 14, and 28) and the Zagreb schedule (“2-1-1”; 2 doses of vaccine on day 0 and 1 dose of vaccine on days 7 and 21)[3]. The Zagreb schedule is only applicable to products approved for use in China, whereas the Essen schedule is suitable for all vaccines and is the most widely used in China; however, the Essen schedule uses more frequent doses and is more time-consuming and expensive than the Zagreb schedule.
The development of processes to optimize rabies vaccine concentration and purification has gradually improved the quality of rabies vaccines. However, further research is necessary to develop immunization schedules that are more efficient and economical than those used today.
The “Rabies vaccines: WHO position paper” published by the World Health Organization (WHO) in 2018 recommends post-exposure immunization schedules such as the simple 4-dose and the Zagreb schedules; the Essen schedule is no longer recommended[4]. Notably, the simple 4-dose schedule (1 dose each on days 0, 3, and 7 and a final dose on any day from 14 to 28) is a simplified version of the Essen method: it uses one dose less than that of the Essen schedule, decreasing the duration of the vaccination programme by 1–2 weeks, improving patient compliance, and reducing the time and financial burden for patients.
According to a review of the literature, the safety, efficacy, and protection induced by the new WHO-recommended simple 4-dose immunization schedule have not been evaluated in China. Specifically, the difference in the immunization effect and the influence of a delayed fourth dose of the simple 4-dose vaccine on immunization potential have not been investigated. Thus, in the context of China, studying the immunological and protective effects of different immunization schedules after rabies exposure is of considerable practical importance.
This study addresses the current gap in the literature by comparing the immune efficacy and immunoprotective effects of different post-exposure immunization schedules at the animal and clinical levels, providing scientific evidence for recommending suitable post-exposure immunization schedules for the Chinese population. The results of this study facilitate updating the post-exposure immunization schedules for rabies vaccination in China.
-
Specific pathogen-free female Kunming mice were purchased from Vital River Laboratory Animal Technology Co., Ltd., Beijing, China, and housed in individual ventilated cages for all experiments. All animals were treated according to China’s regulations and the Animal Experimental Ethical Inspection of the National Institute for Viral Disease Control and Prevention of the Chinese Center for Disease Control and Prevention. Ethical approval was obtained for the animal and human experiments.
This clinical study was conducted with 185 volunteers who received PEP (minors, under 18 years of age; elderly people, over 60 years of age; and adults, aged 18–60 years) at a rabies exposure prevention clinic in Shandong Province. The inclusion criteria for participants were as follows: 1) signed written informed consent to participate in this study, 2) no previous history of rabies vaccination (medical history was collected by a physician, and the individual was excluded if vaccination history was unclear), and 3) post-exposure immunization using the Essen schedule. The exclusion criteria were as follows: 1) incomplete immunization; 2) immunocompromised individuals, that is, patients with acquired immunodeficiency syndrome (CD4 count < 200/mm3), long-term immunosuppressant use for more than 1 month, or long-term corticosteroid therapy (40 mg for more than 2 weeks); and 3) coagulation disorders, such as hemophilia.
-
A purified Vero cell rabies vaccine (PVRV) purchased from Liaoning Chengda Co., Ltd. (production batch number: S20043090) was used in the animal study. The injection amount of mouse vaccine is 1/25 of the human dose[5]. The vaccine used in the clinical trial vaccine was a PVRV manufactured by Guangzhou Norcheng Biological Products Co., Ltd. and Ningbo Rongan Biological Products Co., Ltd.
The national standard reference serum (30 IU/mL) was purchased from the National Institute for Biological Standards and Control (UK). Rabies nucleoprotein antibody labeled with fluorescein isothiocyanate was purchased from Fujirebio Diagnostics, Inc. The challenge virus standard (CVS)-11 was purchased from the National Institutes for Food and Drug Control, China. (CVS)-11 was propagated in BSR cells, and the median lethal dose (LD50) in BALB/c mice was determined using the intramuscular injection route.
-
The mice in each group were strictly isolated, adequate food and water were ensured, and the ambient temperature was maintained at 25–28 °C. During the test, the mice were weighed every 5 d, and the groups were observed for tissue edema at the injection site and for local and systemic reactions in appearance, mental status, behavior, secretions, and excretions.
-
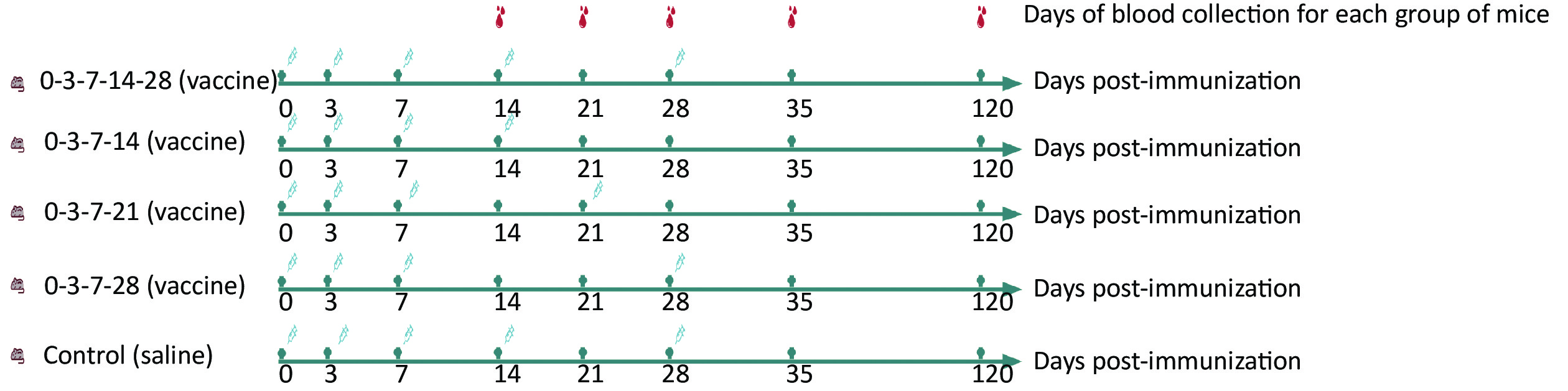
The post-exposure immune efficacy test was performed on the mice after they were divided into five groups (10 mice in each group): 0-3-7-14-28, 0-3-7-14, 0-3-7-21, 0-3-7-28, and control. Next, 0.1 mL vaccine/saline was injected into the medial muscle of the left hind limb of each mouse according to the immunization schedule shown in Figure 1. Blood was collected from each group of mice on days 14, 21, 28, 35, and 120 after the first immunization, and rabies virus neutralizing antibodies (RVNAs) were tested later.
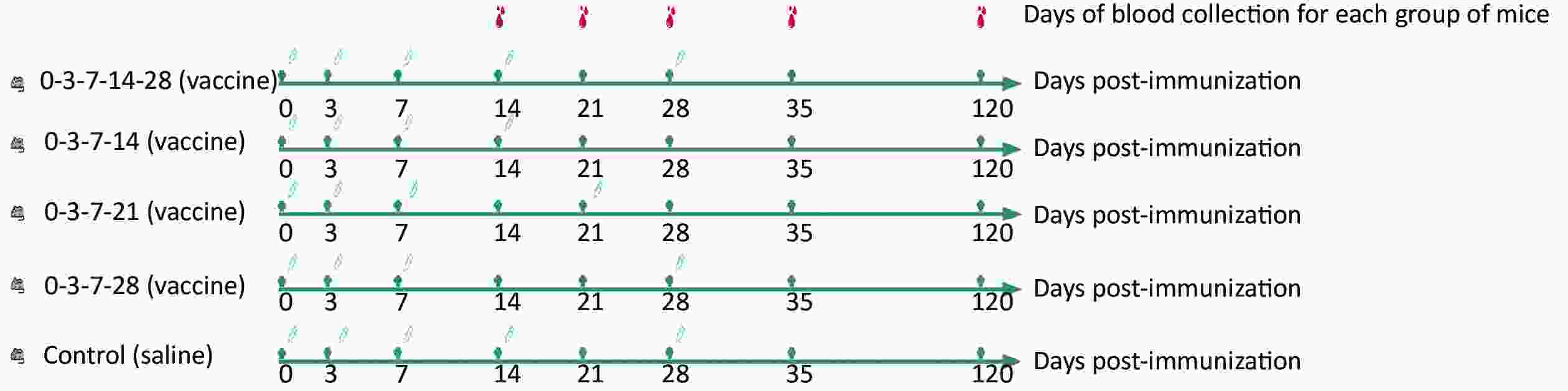
Figure 1. Efficacy evaluation of mouse immunization schedules. Five groups of mice (0-3-7-14-28, 0-3-7-14, 0-3-7-21, 0-3-7-28, and control) immunized with different immunization schedules at 0.1 mL each in the medial muscle of the left hind limb of the mice. Blood was collected from each group on days 14, 21, 28, 35, and 120 after the first immunization, and RVNAs were tested.
-
The post-exposure immune protective test was performed on the mice after they were divided into five groups (10 mice in each group): 0-3-7-14-28, 0-3-7-14, 0-3-7-21, 0-3-7-28, and control. The mice in each group were injected intramuscularly with a lethal dose (LD50) of (CVS)-11 into the right hind limb before immunization, and post-exposure immunization was performed according to different immunization schedules. Clinical symptoms, morbidity, and mortality were observed and recorded daily after the procedure, and brain tissue from dead mice was collected for a direct fluorescent assay (DFA)[6] to confirm whether the mice died from RABV infection.
-
Blood was collected from the orbital arterial plexus of the mice, 0.2 mL at a time, in sterile, dry 1.5 mL centrifuge tubes. The blood samples sat at room temperature for 30 min and then were centrifuged at 2,000 rpm for 10 min at room temperature; next, the serum was separated for RVNAs testing. Brain tissue samples were collected from the mice after death for DFA testing.
-
The rapid fluorescent focus inhibition test was used to determine the RVNAs levels in mice; the procedure and measurement method are described in the literature[3]. A positive RVNAs level of ≥ 0.5 IU/mL was determined according to regulations from the WHO. The positive transfer rate and geometric mean titers (GMTs) of the antibodies at each blood collection point were calculated to assess the efficacy of the immunization schedules.
-
Subjects were immunized using the Essen schedule: 1.0 mL/dose of vaccine on days 0, 3, 7, 14, and 28, each in the deltoid muscle of the upper arm, with an average rabies vaccine potency ≥ 2.5 IU. Venous blood (3–5 mL) was drawn into a nonanticoagulated vacuum tube from subjects on days 28 (14 d after the 4th dose) and 42 (14 d after the 5th dose) after the first immunization, and the serum was isolated for RVNAs levels.
-
The SPSS 22.0 software (SPSS Co., Ltd., Chicago, IL, USA) was used for statistical analysis. Changes in the body weight of the mice before and after the test were analyzed using covariance; the Kruskal‒Wallis test was used to compare the differences between the RVNAs levels of the mice, and differences were considered statistically significant at P < 0.05.
-
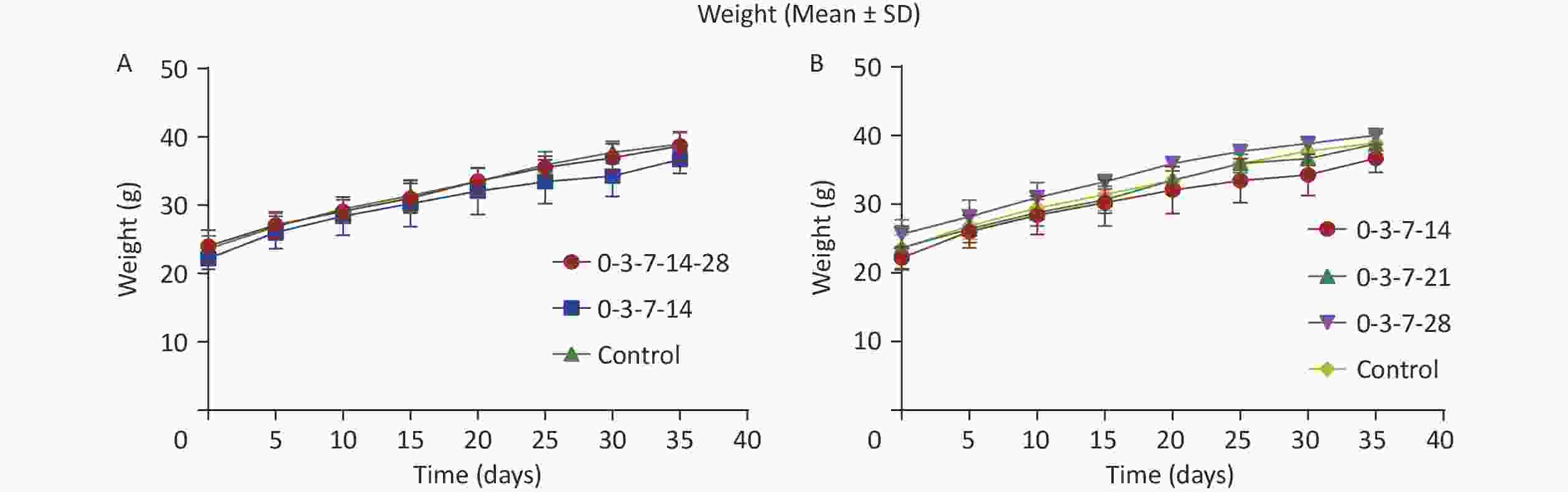
During the experiment, there were no deaths in the experimental or control groups and no edema at the injection site. The mice had a normal appetite, satisfactory growth and development, normal shiny fur, normal clear urine, and gray-brown feces and were lively and responsive. The weight of the mice in each group was measured every 5 d, and the weight changes in the mice in groups 0-3-7-14-28 and 0-3-7-14 are shown in Figure 2A. There was no significant difference in the weight changes of the mice in either group (P > 0.05). The weight changes of the mice at different vaccination times of the 4th dose are shown in Figure 2B. There was also no significant difference in the weight changes of the mice in either of these groups (P > 0.05). The results indicate that different immunization schedules did not have a significant effect on the survival status of the mice.
-
The RVNAs positive conversion rate was 100% in all experimental groups 14 d after the first immunization. The highest RVNAs GMT value was 27.60 IU/mL, and the lowest was 9.26 IU/mL, indicating that all mice in the experimental groups were able to produce an effective immune response 14 d after vaccination. All mice in the control group had RVNAs values of < 0.5 IU/mL throughout the test and were lower than the mice in experimental groups. (P < 0.05), indicating that vaccination provided good immune protection.
The results for the 0-3-7-14-28 and 0-3-7-14 schedules are shown in Figure 3A. The RVNAs levels in the 0-3-7-14-28 and 0-3-7-14 groups increased rapidly from 14 to 35 d after immunization and remained high as the immunization time increased. The RVNAs levels of mice in the 0-3-7-14-28 and 0-3-7-14 groups were 65.92 IU/mL and 32.74 IU/mL, respectively, at 35 d after immunization, showing a significant difference in antibody levels (χ2 = 6.25, P < 0.05); the comparison of the RVNAs levels of mice in each group at 14, 21, 28, and 120 days after immunization showed no significant difference (P > 0.05). The comparison of the RVNAs levels between the groups at 14, 21, 28, and 120 d after immunization showed no significant difference (P > 0.05). The results indicate that the Essen schedule on day 28 resulted in a short-term increase in antibody levels but did not have a significant effect on antibody levels in the medium to long term.
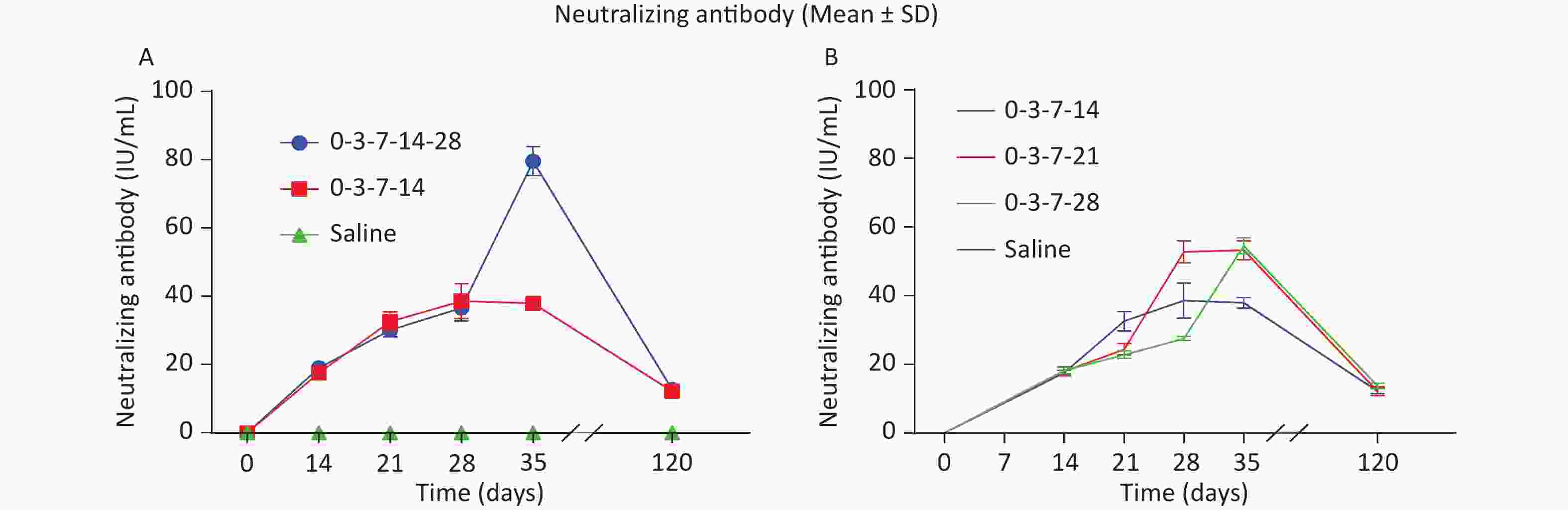
Figure 3. Comparison of immune effect in different groups. (A) Comparison of immune effect between groups 0-3-7-14-28 and 0-3-7-14. (B) Comparison of immune effects of the fourth vaccine dose at different inoculation times. Neutralizing antibody tested 14, 21, 28, 35, and 120 d after the first immunization in each group of mice. Neutralizing antibody values in the graphs are expressed as mean ± SD.
In the comparison of the immune effect of the delayed interval of the fourth dose (groups 0-3-7-14, 0-3-7-21, and 0-3-7-28; Figure 3B), there was no significant difference in the RVNAs levels between the groups at 14, 21, 28, 35 and 120 d after immunization (P > 0.05). These results indicated that there was no statistically significant difference in the RVNAs levels at any time point when comparing the immune effect of the fourth dose of the vaccine at different time points.
-
Clinical symptoms, morbidity, mortality, time of onset, and time of death were observed and recorded daily after inoculation (Figure 4). Mice in each group died from the 8th day onward, and no mice died after the 12th day. In the control group, two mice survived, and eight mice died, with a survival rate of 20%. In each immunization group, four mice survived, and six mice died, with a survival rate of 40%. All mice died before 14 d (4th vaccination), indicating no difference in the protective effect of Essen and the simple 4-dose schedules. DFA was performed on the brain tissues of all dead mice; the results showed specific yellow-green fluorescence in the brain tissue prints of all mice, indicating that all the mice died from RABV infection. The results show no difference between the protective effects of the Essen schedule and the simple 4-dose schedule in mice and that both can provide partial immune protection to mice after exposure.
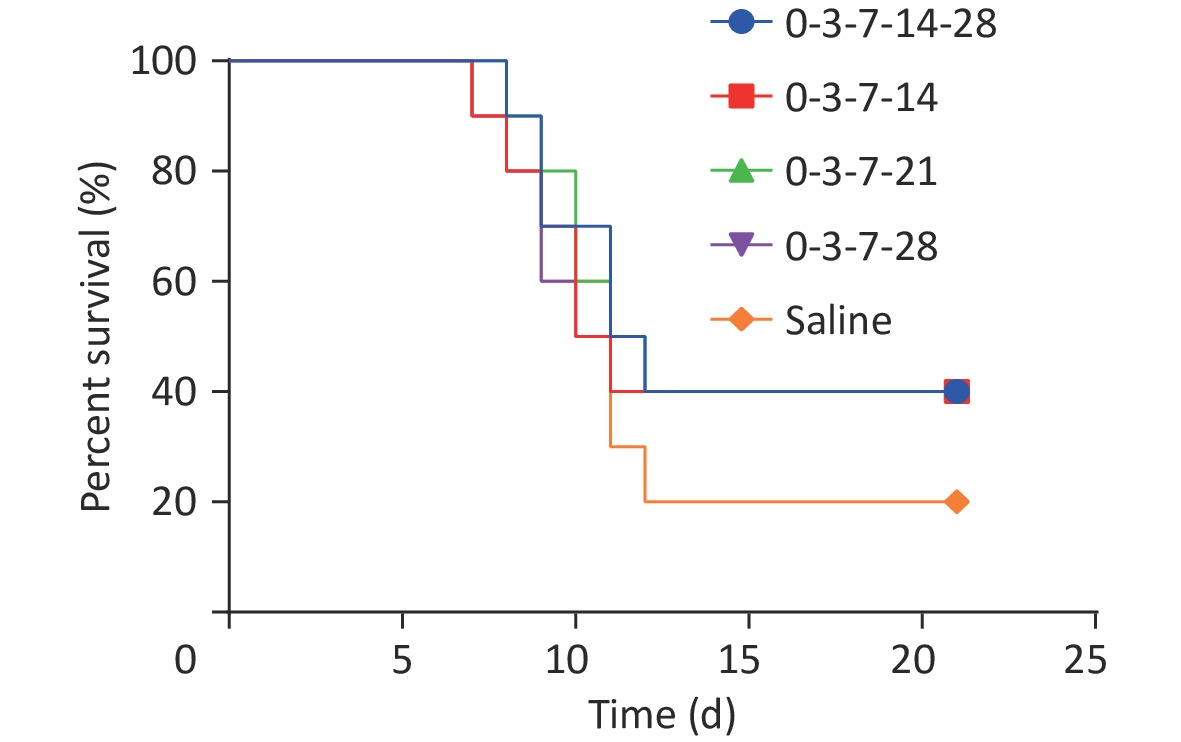
Figure 4. Challenge survival results of PEP with different immunization schedules. Mice in each group died from the 8th day onward, and no mice died after the 12th day. The survival rate of mice in the control group was 20%, and that of mice in each immunization group (groups 0-3-7-14-28, 0-3-7-14, 0-3-7-21, and 0-3-7-28) was 40%.
-
Basic information on the post-exposure immunized volunteers is presented in Table 1. Of the 185 exposed individuals, 98 were male, and 87 were female, with a male-to-female ratio of 1.13:1. Their ages ranged from 2 to 85 years, with the largest number of individuals exposed in the 15–50 age group, accounting for 51.35%. Most individuals were farmers, accounting for 38.38%. Based on the basic patient information, those most exposed to rabies were young and middle-aged individuals, males, and farmers. According to the exposure levels, of the 185 individuals, 49% and 51% were at levels II and III, respectively, and 4.32% were vaccinated with passive immunity methods after exposure. The classification of injured animals revealed that 72.97% were injured by dogs, and 27.57% were injured by cats. None of the injured animals had a clear immunization history, or the immunization history was unknown. Among the vaccines used, 56.22% were Guangzhou Nuocheng, and 43.78% were Ningbo Rongan.
Table 1. Basic information of the participants
Variables Number of cases Composition ratio (%) Sex Male 98 52.97 Female 87 47.03 Age (years) < 15 37 20.00 15– 95 51.35 > 50 53 28.65 Exposure level Class II exposure 91 49.19 Class III exposure 94 50.81 Inoculated with immunoglobulin Yes 8 4.32 No 177 95.68 Animal source of rabies exposure Dog 96 72.97 Cat 51 27.57 Other 38 20.54 Vaccine manufacturers Guangzhou Nuocheng 104 56.22 Ningbo Rongan 81 43.78 -
The positive RVNAs conversion rate was 100% on day 28 after immunization (day 14 after immunization with the fourth dose) and on day 42 (day 14 after immunization with the fifth dose). The GMTs of RVNAs on the 28th and 42nd days were 28.07 IU/mL and 33.05 IU/mL, respectively. The RVNAs levels on the 28th and 42nd days showed no significant difference (χ2 = 0.01, P > 0.05). In addition, the RVNAs levels of volunteers of different sex and age groups with different exposure levels and vaccine manufacturers were compared. The results are shown in Figure 5. There were no significant differences in antibody titers on the 28th or 42nd days in any group (P > 0.05). Moreover, there was no significant difference in antibody levels 14 d after immunization with four injections and 14 d after immunization with five injections. Thus, after the first four doses of the vaccine, the body produced sufficient protective neutralizing antibodies, and the 5th dose of the vaccine did not significantly improve the immune protection effect.
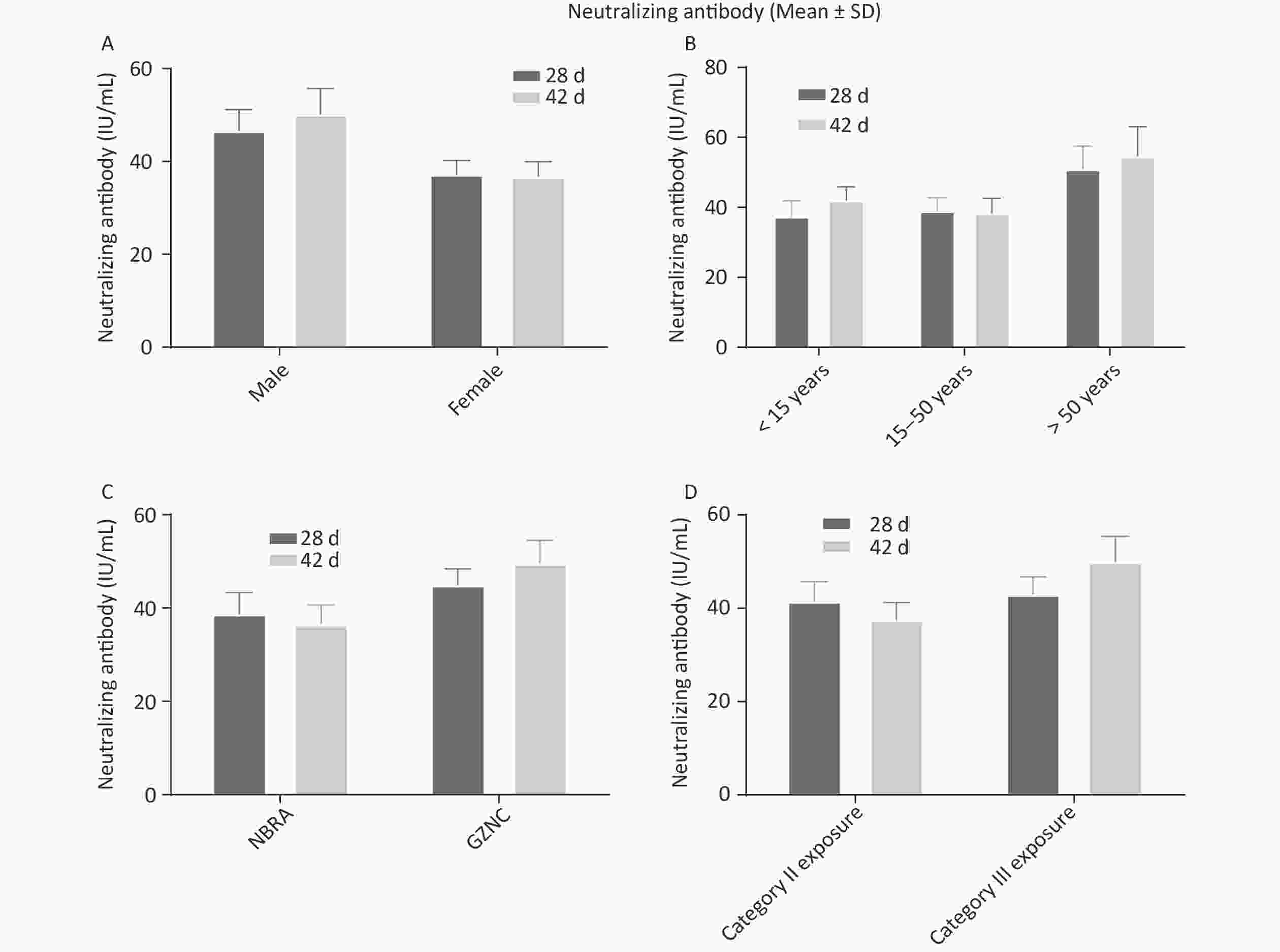
Figure 5. Comparison of RVNAs levels at 28 and 42 d after immunization in different groups. (A) Comparison of RVNAs levels at 28 and 42 d after immunization between the sex groups. (B) Comparison of RVNAs levels at 28 and 42 d after immunization among the age groups. (C) Comparison of RVNAs levels at 28 and 42 d after immunization between the exposure level groups. (D) Comparison of RVNAs levels at 28 and 42 d after immunization between the vaccine manufacturers groups. Neutralizing Antibody values in the graphs are expressed as mean ± SD.
-
The Expert Group on Rabies Immunization Planning in China intended to revise the PEP protocol in the Technical Guidelines for Human Rabies Prevention and Control (2016 version) based on the “Rabies vaccine: WHO position paper”; however, evaluations and research on the safety, effectiveness, and protection of the simple 4-dose immunization schedule in China have not been conducted. In this study, we designed animal and human clinical tests to investigate the safety, effectiveness, and protection of the simple 4-dose schedule, such as whether the immunization effect differs and whether the delayed interval affects the immunization effect if the fourth dose of the simple 4-dose vaccine is delayed, which is not supported by data in China.
In the animal test, the antibody positivity rate of the mice in each group was 100% on the 14th day, and the highest and lowest RVNAs value was 27.60 IU/mL and 9.26 IU/mL, respectively. These results suggest that the mice produced sufficient levels of protective antibodies on the 14th day after immunization. In a related study in Iran, five doses of the human diploid cell vaccine (HDCV) for rabies were administered to rabies-exposed individuals on days 0, 3, 7, 14, and 30, and the antibody levels of all these individuals on the 30th day after the first four doses of the vaccine were higher than the protective level[7].
In our comparison of the immune effects of the Essen schedule and the simple 4-dose schedule, the RVNAs levels of Essen-immunized mice were 65.92 IU/mL on day 35 and 11.32 IU/mL on day 120 after immunization; the RVNAs levels of the mice who were vaccinated according to the simple 4-dose schedule were 32.74 IU/mL on day 35 and 7.71 IU/mL on day 120. Trials have shown that the Essen immunization schedule on day 28 increases antibody levels in the short term but has no significant effect on antibody levels in the medium to long term. In addition, the simple 4-dose schedule had a seroconversion rate of 100% on the 35th day, and the antibody level was much higher than the WHO-recommended criterion for seroconversion (≥ 0.5 IU/mL)[8]. Analysis of the immunization effect demonstrated that the antibody levels produced by the simple 4-dose schedule and the Essen schedule lasted for more than 120 d, indicating that these two immunization schedules had good immunogenicity and immunity persistence, and the fifth injection did not significantly improve immune protection.
In our comparison of the delay interval of the 4th dose of the vaccine (groups 0-3-7-14, 0-3-7-21, and 0-3-7-28), the antibody levels of the mice in each group increased significantly 7–14 d after the fourth dose of the vaccine. However, the RVNAs levels gradually decreased, and the antibody levels of the mice in each group were above the protective level 120 d after immunization, with no significant difference in antibody levels. The fourth dose of the vaccine had no significant effect on the immune response. In general, when the fourth dose of the Essen schedule or a simple 4-dose schedule is administered on any day from 14 to 28, it has a good immune effect. Aoki et al.[9] showed that subjects were vaccinated with HDCV on days 0, 3, 7, 14, and 28 regardless of whether they were injected with rabies immunoglobulin (RIG); there were no significant differences in antibody levels on day 14, and all subjects had protective neutralizing antibodies; antibody levels did not increase after the fourth and fifth doses; and antibody levels (≥ 0.5 IU/mL) persisted for at least 90 d.
In the protection experiment in this study, the survival rate of mice in the control group was 20%, and the survival rate of mice in each immunization group was 40%. This shows that both the Essen schedule and the simple 4-dose schedule provided partial protection to mice after exposure, and there was no significant difference in the protective effect on mice. The results also show that the simple 4-dose schedule is not inferior to the Essen schedule, and the immune memory established after immunization can be maintained in the long term. However, because our study was conducted on mice and the sample size was small, the data collected were limited, and further research is necessary to obtain definite conclusions.
In clinical trials, seropositive conversion rates on days 28 and 42 after immunization using the Essen schedule were 100%, with no significant difference in RVNAs comparisons, and antibody titer values on day 28 were well above 0.5 IU/mL, with adequate levels of protection. The trial showed that the first four doses of the vaccine provided adequate levels of protection, and the fifth dose did not significantly improve immune protection.
This finding is consistent with the results of our animal tests results in the literature[10-11]. A related study[12] showed that all subjects produced adequate antibody levels (GMT 6.8 IU/mL) after the first four doses of purified chicken embryonic cell vaccine for rabies and that antibody titers increased only slightly after the 5th dose of the vaccine, which is consistent with the results of this study. A clinical study in Thailand[13] showed that antibody levels were > 0.5 IU/mL in all those tested 14 d after the first three doses of vaccine and ≥ 2.7 IU/mL after the 4th dose on day 28, and no significant increase in antibody values was observed in individuals vaccinated after the 4th or 5th dose. On day 28 after immunization according to the Essen schedule (regardless of the type of rabies vaccine used), all antibody values reached protective levels (GMTs 6.3–14.5 IU/mL), whereas there was no significant increase in antibody titers after the 5th dose of vaccine. In economically underdeveloped areas, owing to the high cost of rabies vaccines, many patients receive fewer than five doses of the vaccine and do not complete the full course of immunization on time. Studies have shown[14-15] that most rabies cases are due to receiving no vaccination after exposure, no RIG injection, or delayed PEP. No immune failure has been reported because of the omission of the fifth dose of the vaccine. In addition, unpublished epidemiological data from the Centers for Disease Control and Prevention in the United States showed that the immune response provided by the 4-dose vaccine schedule is comparable to that provided by the standard Essen schedule[16].
In conclusion, the 4-dose vaccine schedule can provide good safety and immunogenicity, shorten the immunization time, reduce the burden on patients, and improve compliance. The newly recommended immunization schedules are an influential reference for updating and simplifying immunization schedules in China and provide a guarantee for the early achievement of the goal of eradicating rabies by 2030 set by the WHO and its relevant collaborating organizations. China must update its immunization schedule as soon as possible according to the WHO recommendations, actively explore the feasibility and operability of the newly recommended immunization schedule in the country, actively conduct vaccine effectiveness evaluations, and promote the implementation of rabies vaccine immunization schedules suitable for China’s national conditions.
-
Informed consent was obtained from all participants involved in the study.
doi: 10.3967/bes2024.018
Comparative Study on the Immunogenicity and Efficacy of Different Post-exposure Intramuscular Rabies Vaccination Regimens in China
-
Abstract:
Objective This study aimed to compare the current Essen rabies post-exposure immunization schedule (0-3-7-14-28) in China and the simple 4-dose schedule (0-3-7-14) newly recommended by the World Health Organization in terms of their safety, efficacy, and protection. Methods Mice were vaccinated according to different immunization schedules, and blood was collected for detection of rabies virus neutralizing antibodies (RVNAs) on days 14, 21, 28, 35, and 120 after the first immunization. Additionally, different groups of mice were injected with lethal doses of the CVS-11 virus on day 0, subjected to different rabies immunization schedules, and assessed for morbidity and death status. In a clinical trial, 185 rabies-exposed individuals were selected for post-exposure vaccination according to the Essen schedule, and blood was collected for RVNAs detection on days 28 and 42 after the first immunization. Results A statistically significant difference in RVNAs between mice in the Essen and 0-3-7-14 schedule groups was observed on the 35th day (P < 0.05). The groups 0-3-7-14, 0-3-7-21, and 0-3-7-28 showed no statistically significant difference (P > 0.05) in RVNAs levels at any time point. The post-exposure immune protective test showed that the survival rate of mice in the control group was 20%, whereas that in the immunization groups was 40%. In the clinical trial, the RVNAs positive conversion rates on days 28 (14 days after 4 doses) and 42 (14 days after 5 doses) were both 100%, and no significant difference in RVNAs levels was observed (P > 0.05). Conclusion The simple 4-dose schedule can produce sufficient RVNAs levels, with no significant effect of a delayed fourth vaccine dose (14–28 d) on the immunization potential. -
Key words:
- Rabies /
- Post-exposure immunization /
- Essen regimen /
- RVNAs
The authors declare no competing interests.
注释:1) AUTHOR CONTRIBUTIONS: 2) CONFLICTS OF INTEREST: -
Figure 1. Efficacy evaluation of mouse immunization schedules. Five groups of mice (0-3-7-14-28, 0-3-7-14, 0-3-7-21, 0-3-7-28, and control) immunized with different immunization schedules at 0.1 mL each in the medial muscle of the left hind limb of the mice. Blood was collected from each group on days 14, 21, 28, 35, and 120 after the first immunization, and RVNAs were tested.
Figure 3. Comparison of immune effect in different groups. (A) Comparison of immune effect between groups 0-3-7-14-28 and 0-3-7-14. (B) Comparison of immune effects of the fourth vaccine dose at different inoculation times. Neutralizing antibody tested 14, 21, 28, 35, and 120 d after the first immunization in each group of mice. Neutralizing antibody values in the graphs are expressed as mean ± SD.
Figure 4. Challenge survival results of PEP with different immunization schedules. Mice in each group died from the 8th day onward, and no mice died after the 12th day. The survival rate of mice in the control group was 20%, and that of mice in each immunization group (groups 0-3-7-14-28, 0-3-7-14, 0-3-7-21, and 0-3-7-28) was 40%.
Figure 5. Comparison of RVNAs levels at 28 and 42 d after immunization in different groups. (A) Comparison of RVNAs levels at 28 and 42 d after immunization between the sex groups. (B) Comparison of RVNAs levels at 28 and 42 d after immunization among the age groups. (C) Comparison of RVNAs levels at 28 and 42 d after immunization between the exposure level groups. (D) Comparison of RVNAs levels at 28 and 42 d after immunization between the vaccine manufacturers groups. Neutralizing Antibody values in the graphs are expressed as mean ± SD.
Table 1. Basic information of the participants
Variables Number of cases Composition ratio (%) Sex Male 98 52.97 Female 87 47.03 Age (years) < 15 37 20.00 15– 95 51.35 > 50 53 28.65 Exposure level Class II exposure 91 49.19 Class III exposure 94 50.81 Inoculated with immunoglobulin Yes 8 4.32 No 177 95.68 Animal source of rabies exposure Dog 96 72.97 Cat 51 27.57 Other 38 20.54 Vaccine manufacturers Guangzhou Nuocheng 104 56.22 Ningbo Rongan 81 43.78 -
[1] Taylor LH, Hampson K, Fahrion A, et al. Difficulties in estimating the human burden of canine rabies. Acta Trop, 2017; 165, 133−40. doi: 10.1016/j.actatropica.2015.12.007 [2] Lafon M. Rabies virus receptors. J NeuroVirol, 2005; 11, 82−7. doi: 10.1080/13550280590900427 [3] Chinese Center for Disease Control and Prevention. Technical guideline for human rabies prevention and control (2016). Chin J Viral Dis, 2016; 6, 161−88. (In Chinese [4] World Health Organization. Rabies vaccines: WHO position paper, April 2018 - Recommendations. Vaccine, 2018; 36, 5500−3. doi: 10.1016/j.vaccine.2018.06.061 [5] Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm, 2016; 7, 27−31. doi: 10.4103/0976-0105.177703 [6] Khawplod P, Jaijaroensup W, Sawangvaree A, et al. One clinic visit for pre-exposure rabies vaccination (a preliminary one year study). Vaccine, 2012; 30, 2918−20. doi: 10.1016/j.vaccine.2011.12.028 [7] Bahmanyar M, Fayaz A, Nour-Salehi S, et al. Successful protection of humans exposed to rabies infection. Postexposure treatment with the new human diploid cell rabies vaccine and antirabies serum. JAMA, 1976; 236, 2751−4. doi: 10.1001/jama.1976.03270250019017 [8] Wilde H. Editorial Commentary: Rabies postexposure vaccination: are antibody responses adequate? Clin Infect Dis, 2012; 55, 206-8. [9] Aoki FY, Rubin ME, Friesen AD, et al. Intravenous human rabies immunoglobulin for post-exposure prophylaxis: serum rabies neutralizing antibody concentrations and side-effects. J Biol Stand, 1989; 17, 91−104. doi: 10.1016/0092-1157(89)90032-2 [10] Bernard MC, Boudet F, Pineda-Peña AC, et al. Inhibitory effect of concomitantly administered rabies immunoglobulins on the immunogenicity of commercial and candidate human rabies vaccines in hamsters. Sci Rep, 2022; 12, 6570. doi: 10.1038/s41598-022-10281-1 [11] Rupprecht CE, Briggs D, Brown CM, et al. Evidence for a 4-dose vaccine schedule for human rabies post-exposure prophylaxis in previously non-vaccinated individuals. Vaccine, 2009; 27, 7141−8. doi: 10.1016/j.vaccine.2009.09.029 [12] Wasi C, Chaiprasithikul P, Auewarakul P, et al. The abbreviated 2-1-1 schedule of purified chick embryo cell rabies vaccination for rabies postexposure treatment. Southeast Asian J Trop Med Public Health, 1993; 24, 461−6. [13] Aoki FY, Rubin ME, Fast MV. Rabies neutralizing antibody in serum of children compared to adults following post-exposure prophylaxis. Biologicals, 1992; 20, 283−7. doi: 10.1016/S1045-1056(05)80048-X [14] Sudarshan M K. Assessing burden of rabies in India: WHO sponsored national multicentric rabies survey, 2003. Ind J Comm Med, 2005; 6, 100. [15] Sudarshan MK, Madhusudana SN, Mahendra BJ, et al. Assessing the burden of human rabies in India: results of a national multi-center epidemiological survey. Int J Infect Dis, 2007; 11, 29−35. doi: 10.1016/j.ijid.2005.10.007 [16] Krebs JW, Long-Marin SC, Childs JE. Causes, costs, and estimates of rabies postexposure prophylaxis treatments in the United States. J Public Health Manag Pract, 1998; 4, 56−62. doi: 10.1097/00124784-199809000-00009 -




 下载:
下载:



 Quick Links
Quick Links