-
Despite well-known limitations, mice remain useful as model animals to study tuberculosis (TB) pathogenesis, the basic immune response, the extent of lung pathology as well as efficacy of new drugs against Mycobacterium tuberculosis[1,2]. There are four routes of tuberculosis infection in mice: aerosol generation and exposition, intravenous injection, intranasal administration, and subcutaneous administration[3], and the first two are the most commonly used. The low-dose aerosol model was found to best fit the dissemination of TB cases in the community while intravenous infection is useful to study strain virulence[4]. Intra-nasal and intra-tracheal inoculation techniques have a high degree of variability in the delivery of bacilli into the alveoli and the generation of an aerosol cloud of very small droplet nuclei containing the bacilli was shown to be the most reproducible method[1]. In the case of the intravenous tail injection, the administered doses are higher compared to the aerosol route. The intravenous infection leads to the initial infection established in the lungs followed by the dissemination of the bacteria through the body of the animal. The genetic background of mice plays an important role in susceptibility to the infection and further progression to disease. Intravenous infection can lead to a rapid development of disease and an increased animal lethality after 28 days of the infection but this concerns susceptible C3H/HeJ mice lineage and not resistant C57BL/6 lineage[5]. The latter is resistant to infection and develops severe pulmonary damage after a long period since intravenous infection, around 200 days. Therefore, this design is relevant to study the virulence and is appropriate for observation of the long-term therapy of multi-drug resistant (MDR) M. tuberculosis infection.
M. tuberculosis has a clonal population structure and its different strains/genotypes are notably different in their pathogenic characteristics which in turn may correlate with their enhanced transmission capacity, and wider dissemination hence epidemiologic significance. Previously, a remarkable difference between the major M. tuberculosis strains circulating in Russia was demonstrated in terms of their drug resistance association, in vitro growth features, and in the murine model[6-8]. In particular, genetically related strains of the LAM family demonstrated differences in their virulence and lethality in mice that appeared correlated with dynamic changes in their geographic distribution in European Russia over 20 years[8]. On the other hand, recently described MDR-associated subtypes of the ancient sublineage of the notorious Beijing genotype in the Asian part of Russia demonstrated contrasting virulence and lethality in mice[7] furthermore one of them, a so-called “Buryat” genotype (Beijing 14717-15-cluster) was claimed to be the most lethal Russian strain characterized to date.
Here, we concisely report the most interesting interdependent insights from our just completed study with clinical implications. The C57BL/6 mice were infected with different M. tuberculosis strains; the control group remained untreated while the other group received chemotherapy and the whole experiment lasted for 200 days. Four to six months course of anti-TB drugs is currently recommended by WHO as a short course to treat MDR and XDR-TB and as a result, 85% of TB patients can be cured[9]. Furthermore, this time is sufficiently long to study pathological changes in the lungs of resistant C57BL/6 mice during progression of TB disease, survival of infected animals as well as possible changes in microbial genes under selective pressure of the TB drugs.
We used the C57BL/6 mouse model and intravenous tail injection of the bacterial suspension according to the well-established and validated methodology previously used by us and others to study M. tuberculosis virulence[3,7,10,11]. The M. tuberculosis strains represented the most epidemically relevant global genotypes, Beijing and LAM, and were previously comprehensively characterized by drug susceptibility testing, whole genome sequencing (WGS), and in the same murine model[7,8]. The strains were MDR or pre-extensive drug-resistant (pre-XDR) according to the current WHO definition[9] but differed in virulence and lethality in mice (Table 1).
Table 1. M. tuberculosis strains included in the study
Strain Source and origin Phenotypic drug resistance Genotypic drug resistance and respective mutations Phylogenetic position (lineage, family, spoligotype or cluster) WGS data accession in NCBI SRA 4,542 Sputum (St. Petersburg, Russia) MDR (SHREZ) MDR (SHREZ) RpoB H445D, KatG S315T, PncA D49G, EmbB Q497R, EmbA -12 C > T, rrs 514 A > C, rrs 888 G > A, inhA -12 C > T, eis -12 G > A, Alr L113R Euro-American lineage (Lineage 4), LAM genotype, RD115 LAM-RUS branch, spoligotype SIT266 SRR21776299 7,074 Sputum (St. Petersburg, Russia) Pre-XDR (SHREKOflPAS Eto) pre-XDR (SHRECapFQ) RpoB D435V, KatG S315T, PncA H71R, EmbB M306I, inhA -12 C > T, GyrA D94G, rrs 514 A > C; rrs 1401 A > G Euro-American lineage (Lineage 4), LAM genotype, RD115 LAM-RUS branch, spoligotype SIT252 SRR21776297 396 Sputum (Buryatia, Far East, Russia) MDR (SHREto) MDR (SHR) katG S315T, rpsL K88R, rpoB S450L East-Asian lineage (Lineage 2), Beijing genotype, early ancient sublineage 1 or Asia ancestral 1 (RD181-intact); spoligotype SIT269 SRR18591741 6,691 Sputum (Omsk, West Siberia, Russia) MDR (SHREKCapEto) MDR (SHRE Cap) KatG S315T, KatG I335V, RpoB S450L, RpoC D485N, EmbB Q497R, RpsL K43R, PncA V155A, rrs 1401 A > G East-Asian lineage (Lineage 2), Beijing genotype, early ancient sublineage 2 or Asia ancestral 2 (RD181-deleted); spoligotype SIT1 SRR26387465 Note. LAM, Latin-American Mediterranean; MDR, multidrug resistance; pre-XDR, pre-extensive drug resistance; H, isoniazid; R, rifampin; S, streptomycin; E, ethambutol; K, kanamycin; Ofl, ofloxacin; Cap, capreomycine; Eto, ethionamide; Z, pyrazinamide; PAS, para-Aminosalicylic acid; FQ, fluoroquinolone. All experimental procedures were carried out following the National guidelines “Rules for working with laboratory rodents and rabbits” and were reviewed and approved by the Ethical Committee of the St. Petersburg Research Institute of Phthisopulmonology (Protocol 48.1 of May 20, 2019).
C57BL/6 male mice (weight 16–18 g) were used in all experiments and were obtained from the Andreevka laboratory animal nursery (Moscow region, Russia). The animals were kept under the conditions of a certified animal facility at the St. Petersburg Research Institute of Phthisiopulmonology using NexGen Mouse IVC Cage & Rack system with built-in ventilation and air conditioning system. Before the start of the study, laboratory animals were quarantined for 14 days. A mycobacterial suspension was prepared ex tempore from three-week-old strains. The infecting dose was 106 CFU in 0.2 mL of saline buffer per mouse. A suspension of mycobacteria was inoculated into the lateral tail vein of the animals.
Eight infected animal groups were formed, 20 mice per group, and two groups per each strain. The first infected group was a control one, and the other infected group was receiving an adequate chemotherapy regimen based on the knowledge of the phenotypic and genotypic resistance of the infecting strains (Table 1). The anti-tuberculosis therapy regimen consisted of 4 new-generation or repurposed drugs: moxifloxacin (7 mg/kg), linezolid (10 mg/kg), bedaquiline (6.7 mg/kg for 2 weeks, followed by 3.9 mg/kg for the rest of experiment) and perchlozone (12 mg/kg). This treatment regimen was formulated based on the doses generally recommended to treat TB in humans[12-14]. All drugs were administered orally using atraumatic metal probes, 5 times a week and the drug solutions were prepared on the day of treatment.
The treatment started at day 10 post-infection and lasted for 177 days. After 77 and 177 days of anti-tuberculosis therapy, animals (6 per strain-determined group of mice) were euthanized and examined for different pathology-related characteristics: lung weight coefficient, lung lesions, bacterial load of the lungs, and histology.
Bacterial isolates were cultured from the lungs of the treated mice (one isolate per animal) and euthanized after 77 and 177 days of treatment. Drug susceptibility testing (DST) of the strains was carried out using the method of absolute concentrations (Order No. 109 of the Ministry of Health of the Russian Federation) and/or using the modified proportion method and automated system BACTEC MGIT 960 system (Becton Dickinson, Sparks, Md.) according to the manufacturer’s instructions and WHO recommendations[9]. The critical drug concentrations used were 1.0 μg/mL for streptomycin, 0.1 μg/mL for isoniazid, 5.0 μg/mL for ethambutol, 1.0 μg/mL for rifampicin, 100 μg/mL for pyrazinamide, 1.0 μg/mL for amikacin, 2.5 μg/ mL for capreomycin, 2.0 μg/mL for ofloxacin, 5 μg/mL for ethionamide, 0.25 and 1.0 μg/mL for moxifloxacin, 1.0 μg/mL for linezolid, and 1.0 μg/mL for bedaquiline. Drug susceptibility testing was not performed for perchlozone because no critical concentrations have been approved and perchlozone susceptibility testing has not yet been implemented[15].
Bacterial DNA was extracted using the Cetyltrimethylammonium bromide-based method and submitted to the whole genome sequencing (WGS). Whole genome, paired-end sequencing on the HiSeq2500 platform was done using NEBNext Ultra and PhiX Control v3 kits (Illumina). DNA libraries were prepared using ultrasound DNA fragmentation and NEBNext Ultra DNA Library Prep Kit for Illumina (New England Biolabs). Data for the M. tuberculosis sequenced genomes (fastq files) were deposited in the NCBI Sequence Read Archive (project number PRJNA1028254).
SAM-TB online tool (https://samtb.uni-medica.com/index) was used for SNP calling and genotypic detection of drug resistance. MDR, pre-XDR, and XDR phenotypes were defined according to the updated WHO definitions: MDR are strains resistant to isoniazid and rifampicin; pre-XDR-resistant to isoniazid, rifampicin, fluoroquinolone; XDR-resistant to isoniazid, rifampicin, fluoroquinolone plus bedaquiline and/or linezolid. Geneious 9.0 package (Biomatters Ltd) was additionally used for mapping the reads to the genome of reference strain H37Rv (NC_00962.3) and analysis of the drug resistance genes for possible emergence of mutant alleles.
In brief, after 77 days of anti-TB therapy, in comparison with the respective infected control groups, a decrease was recorded in lung weight coefficients, bacterial load of the lungs, and abundance of lung lesions. A complete treatment course of 177 days significantly reduced the contamination of the lungs with M. tuberculosis and the prevalence of tuberculous lung damage decreased in all treated groups.
We note that M. tuberculosis strains were isolated from the lungs of euthanized animals even after 5.5 months of treatment. From each strain-determined group, there were 6 mice/isolates analyzed per each time-point except for the group infected with low-virulent strain 6,691 where 4 isolates were recovered after 5.5 months of treatment. In this sense, 177 days of uninterrupted and adequate chemotherapy could not eradicate the pathogen and provide sterile immunity but this is not unexpected. A recent human TB study demonstrated this and highlighted how ambitious (unrealistic) the WHO plan to eliminate tuberculosis is. Patterson et al.[16] quantified viable aerosolized M. tuberculosis from TB clinic attendees in the high-burden area in South Africa and M. tuberculosis was detected in bioaerosols in a notable proportion (~30%) of confirmed TB patients after completion of the standard 6-month treatment.
We further performed DST and WGS of the M. tuberculosis isolates recovered from the lungs of euthanized treated mice after 2 and 5.5 months of treatment. The phenotypic DST revealed that the infecting strains did not change the initial resistance profiles (Table 1) and remained susceptible to the administered drugs moxifloxacin, bedaquiline, and linezolid.
The generated short sequencing reads (fastq files) were analyzed using the SAM-TB web-based pipeline to detect possible mutations in the M. tuberculosis genes known to be associated with resistance to the administered drugs. This online tool permits a comprehensive genotypic drug-resistance prediction for 17 antituberculosis drugs based on the TB Profiler tool and CRyPTIC Consortium and 100 000 Genomes Project followed by further correction and curation[17].
We additionally mapped the sequencing reads to the H37Rv reference genome with Geneious package. We checked manually the reads mapped to the genes gyrA, and gyrB genes (fluoroquinolones resistance), rplC, rrs, rrl (linezolid resistance), Rv0678, atpE, pepQ, and Rv1979c (bedaquiline resistance). To increase the sequencing depth, we pooled together isolates from each strain-determined group together and as a result, the sequencing depth was ~1600x. No mutant alleles were identified in linezolid and bedaquiline resistance genes even at a negligible percentage. Regarding fluoroquinolone resistance, no mutations in gyrA/gyrB were found in isolates from mice infected with LAM 4542 and both Beijing strains. A gyrA mutation (D94G) was initially present in one LAM strain 7074 but no additional gyrA/gyrB mutations emerged in this strain during the experiment. Summing up, no mutations associated with resistance to the administered drugs emerged during 5.5 months of treatment even at a minor percentage. This finding is remarkable given the known emergence of resistance mutations in consecutive isolates recovered from human patients during not only long-term but also relatively short-term treatment, e.g. with bedaquiline[18,19]. However, in real life, unfortunately, human patients may and do have multiple episodes of interrupted chemotherapy for any reason[9,18-21], and this makes a great difference compared to the fully compliant mice receiving scientifically sound adequate chemotherapy.
Natural death of mice was also recorded during the experiment. The survival analysis showed that no mice died during 200 days in three of the four strain-determined animal groups that received chemotherapy (Figure 1). Only in one group of mice infected with hypervirulent and highly-lethal strain 396, the lethal cases were recorded but less frequently compared to the untreated group.
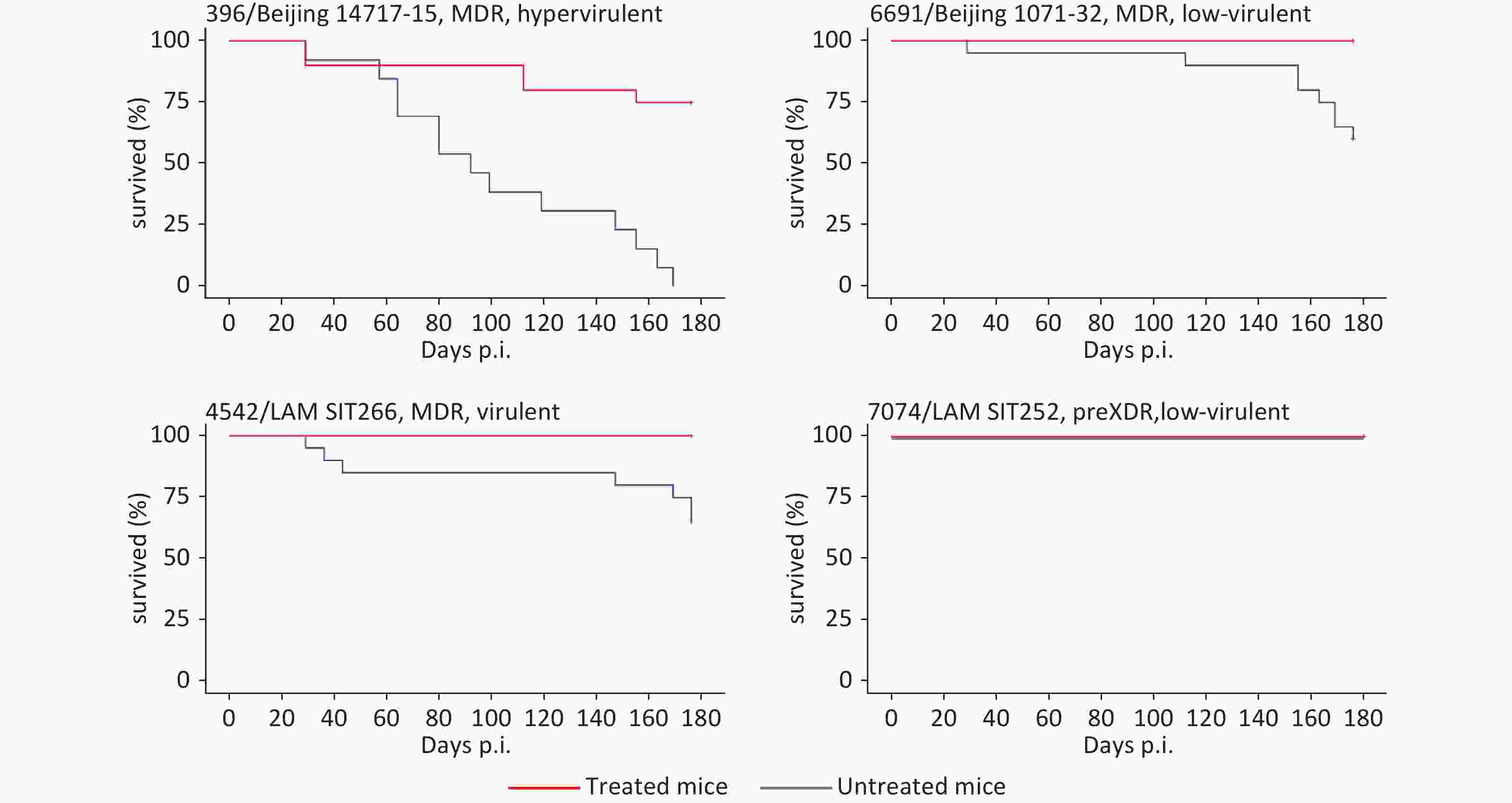
Figure 1. Survival curves for eight mouse groups infected with different M. tuberculosis strains and treated or not treated with anti-TB chemotherapy. LAM, Latin-American Mediterranean; MDR, multidrug resistance; pre-XDR, pre-extensive drugresistance.
To conclude, the correct chemotherapy regimen and complete compliance resulted in efficient treatment, the absence of emerging resistance to the administered drugs, and drastically reduced lethality in all treated groups of animals. Our findings highlight that early diagnosis including detection of resistance determinants, hence early choice of correct chemotherapy regimen and patient compliance are the key factors to control TB. Indirectly, this implies that the WHO resistance mutation catalogue[22] should be further updated and improved to provide the highest sensitivity and specificity of the early genotypic detection of drug resistance in clinical samples from TB patients.
At the same time, lethal cases were still observed in the mice infected with hypervirulent strain 396 (Beijing 14717-15 subtype), in spite of adequate treatment. This implies that truly personalized medicine should consider not only drug resistance but other pathobiological properties of M. tuberculosis strains to begin with virulence. In this view, research focused on the so-called anti-virulence drugs is most relevant and justified.
-
All experimental procedures were approved by the Ethical Committee of the St. Petersburg Research Institute of Phthisopulmonology (Protocol 48.1 of May 20, 2019).
doi: 10.3967/bes2024.084
What Mice Can Teach Us about How to Stop Drug-Resistant Tuberculosis: Correct Chemotherapy Regimen and Patient Compliance are the Key
-
Igor Mokrousov: Funding acquisition, conceptualization, formal analysis, and writing-original Draft; Tatiana Vinogradova: Conceptualization, supervision, investigation, and writing-original draft; Marine Dogonadze, Maria Vitovskaya, Natalia Zabolotnykh, Sergei Chekrygin, and Anna Vyazovaya: Investigation.
Authors declare that no conflict of interest exist.
注释:1) Author Contributions: 2) Conflict of Interest: -
Table 1. M. tuberculosis strains included in the study
Strain Source and origin Phenotypic drug resistance Genotypic drug resistance and respective mutations Phylogenetic position (lineage, family, spoligotype or cluster) WGS data accession in NCBI SRA 4,542 Sputum (St. Petersburg, Russia) MDR (SHREZ) MDR (SHREZ) RpoB H445D, KatG S315T, PncA D49G, EmbB Q497R, EmbA -12 C > T, rrs 514 A > C, rrs 888 G > A, inhA -12 C > T, eis -12 G > A, Alr L113R Euro-American lineage (Lineage 4), LAM genotype, RD115 LAM-RUS branch, spoligotype SIT266 SRR21776299 7,074 Sputum (St. Petersburg, Russia) Pre-XDR (SHREKOflPAS Eto) pre-XDR (SHRECapFQ) RpoB D435V, KatG S315T, PncA H71R, EmbB M306I, inhA -12 C > T, GyrA D94G, rrs 514 A > C; rrs 1401 A > G Euro-American lineage (Lineage 4), LAM genotype, RD115 LAM-RUS branch, spoligotype SIT252 SRR21776297 396 Sputum (Buryatia, Far East, Russia) MDR (SHREto) MDR (SHR) katG S315T, rpsL K88R, rpoB S450L East-Asian lineage (Lineage 2), Beijing genotype, early ancient sublineage 1 or Asia ancestral 1 (RD181-intact); spoligotype SIT269 SRR18591741 6,691 Sputum (Omsk, West Siberia, Russia) MDR (SHREKCapEto) MDR (SHRE Cap) KatG S315T, KatG I335V, RpoB S450L, RpoC D485N, EmbB Q497R, RpsL K43R, PncA V155A, rrs 1401 A > G East-Asian lineage (Lineage 2), Beijing genotype, early ancient sublineage 2 or Asia ancestral 2 (RD181-deleted); spoligotype SIT1 SRR26387465 Note. LAM, Latin-American Mediterranean; MDR, multidrug resistance; pre-XDR, pre-extensive drug resistance; H, isoniazid; R, rifampin; S, streptomycin; E, ethambutol; K, kanamycin; Ofl, ofloxacin; Cap, capreomycine; Eto, ethionamide; Z, pyrazinamide; PAS, para-Aminosalicylic acid; FQ, fluoroquinolone. -
[1] Ordway DJ, Orme IM. Animal models of mycobacteria infection. Curr Protoc Immunol, 2011; Chapter 19: Unit19. 5. [2] De Groote MA, Gilliland JC, Wells CL, et al. Comparative studies evaluating mouse models used for efficacy testing of experimental drugs against Mycobacterium tuberculosis. Antimicrob Agents Chemother, 2011; 55, 1237−47. doi: 10.1128/AAC.00595-10 [3] Soldevilla P, Vilaplana C, Cardona PJ. Mouse models for Mycobacterium tuberculosis pathogenesis: show and do not tell. Pathogens, 2023; 12, 49. [4] Caceres N, Llopis I, Marzo E, et al. Low dose aerosol fitness at the innate phase of murine infection better predicts virulence amongst clinical strains of Mycobacterium tuberculosis. PLoS One, 2012; 7, e29010. doi: 10.1371/journal.pone.0029010 [5] Chackerian AA, Perera TV, Behar SM. Gamma interferon-producing CD4+ T lymphocytes in the lung correlate with resistance to infection with Mycobacterium tuberculosis. Infect Immun, 2001; 69, 2666−74. doi: 10.1128/IAI.69.4.2666-2674.2001 [6] Fursov MV, Shitikov EA, Bespyatykh JA, et al. Genotyping, assessment of virulence and antibacterial resistance of the Rostov strain of Mycobacterium tuberculosis attributed to the central Asia outbreak clade. Pathogens, 2020; 9, 335. doi: 10.3390/pathogens9050335 [7] Vinogradova T, Dogonadze M, Zabolotnykh N, et al. Extremely lethal and hypervirulent Mycobacterium tuberculosis strain cluster emerging in Far East, Russia. Emerg Microbes Infect, 2021; 10, 1691−701. doi: 10.1080/22221751.2021.1967704 [8] Mokrousov I, Vinogradova T, Dogonadze M, et al. A multifaceted interplay between virulence, drug resistance, and the phylogeographic landscape of Mycobacterium tuberculosis. Microbiol Spectr, 2023; 11, e0139223. doi: 10.1128/spectrum.01392-23 [9] WHO. Global Tuberculosis Report 2023. Geneva: World Health Organization; 2023. [10] Stukova MA, Sereinig S, Zabolotnyh NV, et al. Vaccine potential of influenza vectors expressing Mycobacterium tuberculosis ESAT-6 protein. Tuberculosis, 2006; 86, 236−46. doi: 10.1016/j.tube.2006.01.010 [11] Marquis JF, LaCourse R, Ryan L, et al. Disseminated and rapidly fatal tuberculosis in mice bearing a defective allele at IFN regulatory factor 8. J Immunol, 2009; 182, 3008−15. doi: 10.4049/jimmunol.0800680 [12] WHO. WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment. Geneva: World Health Organization; 2019. [13] Yablonsky PK. National clinical guidelines. GEOTAR-Media. 2016, 240 p. [14] Pavlova MV, Vinogradova TI, Zabolotnykh NV, et al. Prospects for the use of new generation of anti-tuberculosis drugs in treatment of drug-resistant tuberculosis. Rev Clin Pharm Drug Ther, 2020; 18, 115−21. (In Russian [15] Ushtanit A, Mikhailova Y, Krylova L, et al. Perchlozone resistance in clinical isolates of Mycobacterium tuberculosis. Antibiotics, 2023; 12, 590. doi: 10.3390/antibiotics12030590 [16] Patterson B, Dinkele R, Gessner S, et al. Aerosolization of viable Mycobacterium tuberculosis bacilli by tuberculosis clinic attendees independent of sputum-Xpert ultra status. Proc Natl Acad Sci USA, 2024; 121, e2314813121. doi: 10.1073/pnas.2314813121 [17] Yang TT, Gan MY, Liu QY, et al. SAM-TB: a whole genome sequencing data analysis website for detection of Mycobacterium tuberculosis drug resistance and transmission. Brief Bioinform, 2022; 23, bbac030. doi: 10.1093/bib/bbac030 [18] Mokrousov I, Akhmedova G, Molchanov V, et al. Frequent acquisition of bedaquiline resistance by epidemic extensively drug-resistant Mycobacterium tuberculosis strains in Russia during long-term treatment. Clin Microbiol Infect, 2021; 27, 478−80. doi: 10.1016/j.cmi.2020.08.030 [19] Perumal R, Khan A, Naidoo K, et al. Mycobacterium tuberculosis intra-host evolution among drug-resistant tuberculosis patients failing treatment. Infect Drug Resist, 2023; 16, 2849−59. doi: 10.2147/IDR.S408976 [20] Smith SE, Ershova J, Vlasova N, et al. Risk factors for acquisition of drug resistance during multidrug-resistant tuberculosis treatment, Arkhangelsk Oblast, Russia, 2005-2010. Emerg Infect Dis, 2015; 21, 1002−11. doi: 10.3201/eid2106.141907 [21] Al-Hajjaj MS, Al-Khatim IM. High rate of non-compliance with anti-tuberculosis treatment despite a retrieval system: a call for implementation of directly observed therapy in Saudi Arabia. Int J Tuberc Lung Dis, 2000; 4, 345−9. [22] Walker TM, Miotto P, Köser CU, et al. The 2021 WHO catalogue of Mycobacterium tuberculosis complex mutations associated with drug resistance: a genotypic analysis. Lancet Microbe, 2022; 3, e265−73. doi: 10.1016/S2666-5247(21)00301-3 -




 下载:
下载:



 Quick Links
Quick Links