-
Sepsis is an inflammatory disease resulting from a dysregulated host response to infection that may lead to multiple organ failure and is commonly observed in patients with severe trauma or infectious diseases. Sepsis has become a major global public health issue due to its high incidence and fatality. It not only causes an excessive inflammatory response, but also leads to immune system dysfunction[1]. As a vital organ with diverse immune functions, the spleen plays an indispensable role in the body's resistance to sepsis. Current therapeutic strategies for sepsis include anti-infective therapy, fluid resuscitation, vasoactive medications, and symptomatic supportive care. Timely and effective control of the source of infection is the core of sepsis treatment, and fluid resuscitation and increasing vascular tone are the basis of sepsis treatment. The treatment of sepsis has evolved from simple anti-infective therapy in the early days to comprehensive therapy involving the protection and support of multiple organ systems. In the future, with the progress in biomedical technology and an in-depth understanding of the mechanism of sepsis, personalized treatment strategies and innovative therapies are expected to further improve the prognosis of patients with sepsis. Studies have found that natural small-molecule compounds such as polyphenols, flavonoids, and terpenoids play important roles in the treatment of sepsis and have gradually become the focus of research[2].
Given the advantages of multiple targets and few or no side effects, natural compounds have become an important source for developing new therapies for sepsis. Monotropein (MON) is the main iridoid glycoside component of Morinda officinalis and has a variety of pharmacological activities, ranging from anti-inflammatory to antioxidant and anti-apoptotic[3]. Several studies have confirmed that MON exhibits organ-protective properties in various disease models. For example, MON attenuates liver injury secondary to chronic colitis through the suppression of the toll-like receptor 4 (TLR4)/ nuclear factor kappa-B (NF-κB)/NLR family pyrin domain containing protein 3 (NLRP3) axis[4]. MON alleviates acute kidney damage induced by cisplatin via suppressing inflammation, oxidative stress, and apoptosis[5]. However, the effects of MON on sepsis-induced spleen injury have not been clarified.
The emergence and progression of sepsis usually involves a complex systemic inflammatory response and immune dysfunction, which are closely linked to multiple pathophysiological variations in the body's systems and organs. Currently, the commonly used sepsis models include the cecum ligation and puncture (CLP) and lipopolysaccharide (LPS) models, which can be employed to explore the pathogenesis of sepsis. CLP is the best model for replicating clinical sepsis and better reflects bidirectional changes in immunity and hemodynamics during the development of sepsis. LPS is the main endotoxin of gram-negative bacteria and induces acute inflammation via stimulating the generation of proinflammatory cytokines by host cells, thereby eliciting complex immune and inflammatory responses[6]. In the present study, a mouse model of sepsis-related splenic injury was established using CLP to investigate whether MON exerts a protective effect on the spleen. To further elucidate the underlying molecular mechanism of MON, we established an in vitro sepsis model using LPS-stimulated RAW264.7 macrophages.
Six-week-old male BALB/c mice, weighing 20–25 g, were purchased from the Animal Experiment Center of Yangzhou University. The mice were reared under pathogen-free conditions, and the animal rearing environment was kept at a temperature of 25 ± 2 °C, relative humidity of 55% ± 15%, and light-dark alternating time for 12 h/12 h. All mice were fed pathogen-free food and drinking water and treated with disinfection and sterilization. All animal experiments were reviewed and approved by the Animal Ethics Committee of Jiangsu Ocean University. Forty mice were randomly assigned to four groups (10 mice per group): Sham, CLP, CLP+MON, and CLP+Dexamethasone (DEX) groups. After the mice were anesthetized, a mid-abdominal incision was made to expose the cecum and was ligated at the half distal end of the cecum. A small amount of feces was extruded following puncture, then the cecum was placed back into the abdominal cavity and the incision was sutured. In the Sham group, only the abdomen was incised, and the incision was sutured without cecal ligation or puncture. The drug or saline was injected intraperitoneally once daily for five consecutive days after CLP surgery. The CLP+MON group mice were administrated with MON (20 mg/kg), the CLP+DEX group mice were treated with DEX (1 mg/kg), and the remaining mice were injected with an equal amount of saline. All mice were euthanized on day 5 after CLP surgery and spleen tissues were collected for subsequent experiments.
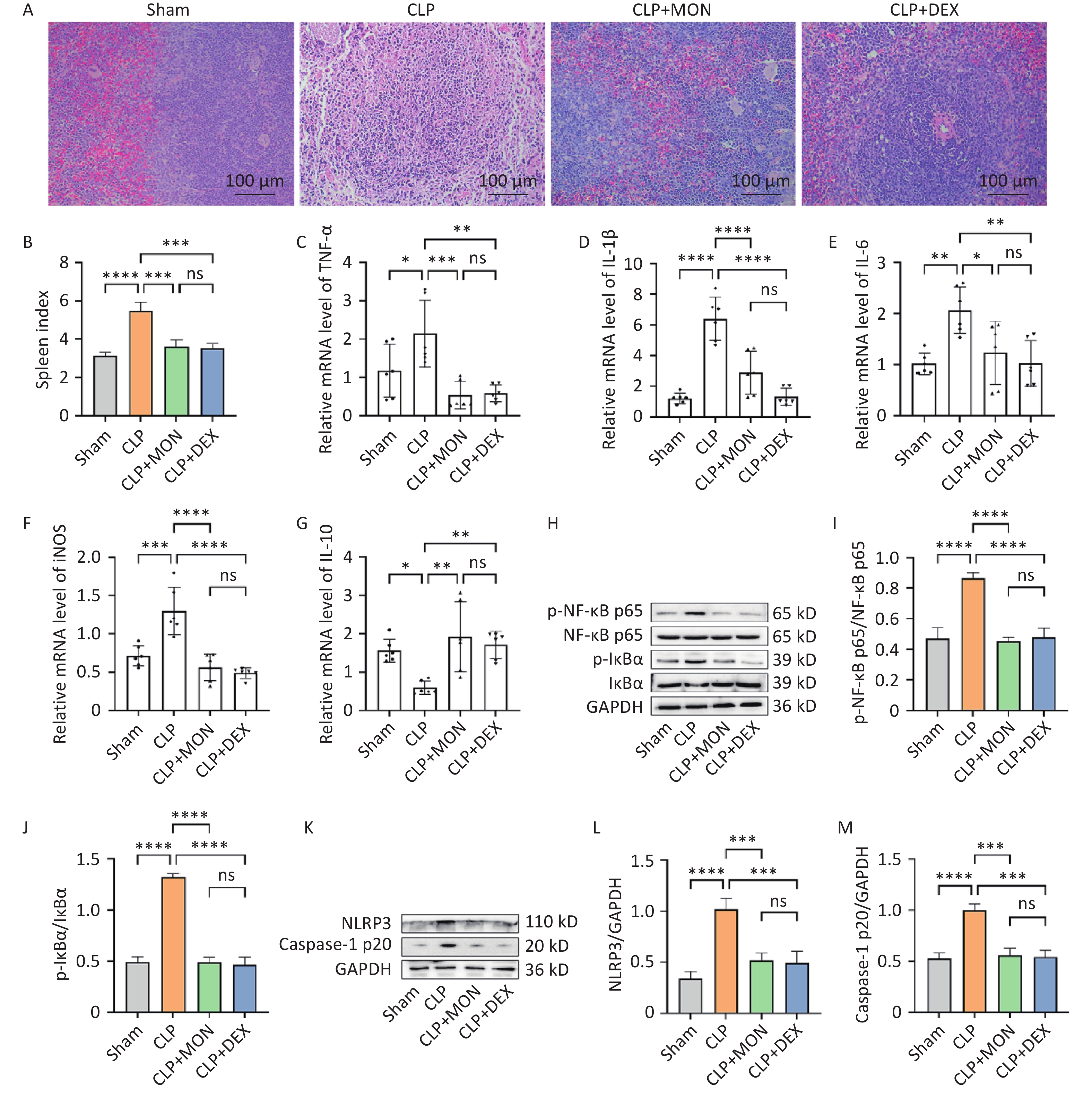
Hematoxylin-Eosin (HE) staining revealed that mice in the Sham group had a normal spleen tissue structure, whereas the spleen structure of the CLP group was obviously damaged, and the boundary between the red and white medulla was blurred. Splenic histopathological damage was remarkably improved in the MON and DEX treatment groups (Figure 1A). The spleen index was notably increased in the CLP group, whereas it was markedly decreased in the MON-treated group (Figure 1B). The spleen plays an instrumental role in the regulation of inflammation by controlling the release of cytokines to maintain a balanced immune status. Inflammatory response is one of the primary causes of organ failure due to sepsis, and proinflammatory cytokines are usually elevated during sepsis. IL-10 is a key anti-inflammatory cytokine that inhibits the release of proinflammatory cytokines. The actual outcome of the inflammatory response depends on the balance between pro- and anti-inflammatory cytokines. The results showed that the mRNA levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and inducible nitric oxide synthase (iNOS) were significantly lower, and the mRNA level of IL-10 was markedly higher in the MON treatment group than in the CLP group (Figure 1C–G). Thus, MON exerts its anti-inflammatory effects by inhibiting the release of pro-inflammatory cytokines and increasing the production of anti-inflammatory cytokines.

Figure 1. Effect of MON on spleen pathological injury and inflammatory response in CLP-induced septic mice. (A) Representative images of HE staining of spleen tissues. (B) Spleen index of mice. (C–G) The mRNA levels of TNF-α, IL-1β, IL-6, iNOS and IL-10 were examined via RT-qPCR (n = 6). (H) Western blot detection of the expressions of NF-κB pathway-related proteins. (I–J) Gray-scale value analysis of p-NF-κB p65/NF-κB p65 and p-IκBα/IκBα (mean ± SD, n = 3). (K–M) Protein expressions of NLRP3 and Caspase-1 p20 were detected and quantified (mean ± SD, n = 3). Comparisons of differences between groups were indicated by *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; ns, not significant.
NF-κB and NLRP3 inflammasome are important inflammation-related signaling pathways that play key roles in intrinsic immunity, cytokine release, and inflammatory responses. Dysregulation of NF-κB activity can cause a cytokine storm, leading to multi-organ failure[7]. The NLRP3 inflammasome regulates the expression of inflammatory cytokines, which plays a crucial role in the development of sepsis. Studies have shown that inhibiting the overactivation of the NLRP3 inflammasome can attenuate sepsis-induced cardiomyopathy[8]. Our study found that NF-κB p65 and IκBα protein phosphorylation levels were clearly higher and IκBα degradation was increased in the CLP group compared to the Sham group (Figure 1H). Compared with the CLP group, p-NF-κB p65/NF-κB p65 and p-IκBα/IκBα levels were remarkably decreased in the MON-treated group, suggesting that MON reduced the activation of the NF-κB pathway (Figure 1I–J). In addition, MON treatedment inhibited the protein expressions of NLRP3 and Caspase-1 p20 compared to the CLP group (Figure 1K–M). In summary, the anti-inflammatory action of MON may be associated with the suppression of the NF-κB/NLRP3 axis.
Oxidative stress is a key pathogenic mechanism of multiple organ dysfunction caused by sepsis. Prolonged exposure to high levels of reactive oxygen species (ROS) leads to an imbalance in the body's redox system. Intracellular ROS levels are positively correlated with the degree of oxidative stress. Dihydroethidium (DHE) staining showed that the ROS content in the CLP group was markedly elevated compared to that in the Sham group. In contrast, MON and DEX administration alleviated CLP-induced ROS accumulation (Figure 2A–B). Malondialdehyde (MDA) production is closely related to the extent of oxidative stress in cells and its content reflects the degree of lipid peroxidation and cellular damage. Catalase (CAT) and glutathione (GSH) are the major antioxidants in the body, which can protect cells from oxidative injuries. Total antioxidant capacity (T-AOC) reflects the comprehensive ability of antioxidants in organisms to scavenge free radicals and resist oxidative stress, making it an influential indicator for assessing the entire function of the antioxidant defense system. In this study, we confirmed that MON exerts an anti-oxidative stress effect by increasing the levels of CAT, GSH, and T-AOC and suppressing the overproduction of MDA (Figure 2C–F). Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor that plays a crucial role in antioxidant stress response. Under normal conditions, Nrf2 binds to Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm and is degraded via the ubiquitin-proteasome pathway. When cells are stimulated by oxidative stress, the interaction between Nrf2 and Keap1 is disrupted, and Nrf2 is released into the nucleus to induce the expression of antioxidant genes, thereby protecting cells against oxidative stress damage[9]. As presented in Figure 2G–J, compared to the Sham group, the expression levels of Keap1 were markedly enhanced, the expression levels of Nrf2 in the nucleus were decreased, and the Nrf2 levels in the cytoplasm were elevated in the CLP group. However, MON administration group increased the protein expressions of Nrf2 in the nucleus, indicating that MON activated the Nrf2 pathway, thereby alleviating splenic oxidative stress injury.

Figure 2. Effect of MON on oxidative stress in the spleen of CLP-induced septic mice. (A) ROS contents were detected by DHE staining. Blue signals indicated DAPI-stained nuclei and red signals indicated ROS production. (B) Quantitative analysis of ROS fluorescence intensity in spleen tissues (mean ± SD, n = 3). (C–F) Absorbance of MDA, CAT, GSH and T-AOC was measured at 532, 405, 412, and 520 nm, respectively, using a multifunctional microplate detector, and the content of each indicator was calculated (n = 6). (G–J) Protein expressions of Keap1, Nrf2 in the nucleus and Nrf2 in the cytoplasm were examined and quantified (n = 3). Comparisons of differences between groups were indicated by *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; ns, not significant.
Apoptosis is a key factor in the pathophysiological changes of organ damage associated with sepsis. The findings showed that the number of apoptotic cells stained with TUNEL in the CLP group was markedly higher than in the Sham group. Interestingly, apoptosis was significantly alleviated in the MON and DEX treatment groups (Figure 3A–B). Excessive inflammatory responses and oxidative stress trigger the mitochondrial apoptotic pathway and accelerate organ failure. The results indicated that compared with the Sham group, the expressions of Bcl-2 associated X protein (Bax), cleaved Caspase-3, and Cytochrome C (Cyt C) were obviously elevated, whereas the expressions of B-cell lymphoma-2 (Bcl-2) were decreased in the CLP group (Figure 3C–G). However, MON treatment reversed this change, indicating that MON inhibits apoptosis mediated by the mitochondrial pathway. Macrophages are important immune cells that defend against microbial invasion and clear pathogens and play an important role in various inflammatory diseases, including sepsis. Therefore, we selected RAW264.7 macrophages to create an in vitro model of sepsis. Nrf2 has been shown to benefit the maintenance of mitochondrial structure and function[10]. ML385 is an Nrf2-specific inhibitor that reduces Nrf2 transcriptional activity. CCK-8 results showed that 0–20 μmol/L was the safe concentration range of MON for RAW264.7 cells (Figure 3H). The results showed that the protein expressions of Bax, cleaved Caspase-3, and Cyt C were significantly higher, whereas Bcl-2 and nuclear Nrf2 expressions were markedly lower in the LPS group than in the control group. However, the opposite trend was observed in the LPS+MON group. Compared to the LPS+MON group, the addition of ML385 reversed the protective action of MON on RAW264.7 cells (Figure 3I–N). Thus, the suppressive effect of MON on apoptosis may be partially dependent on the activation of the Nrf2 pathway.

Figure 3. Effect of MON on apoptosis in CLP-induced mice spleen tissues and LPS-treated RAW 264.7 cells. (A) Splenic cell apoptosis was detected by TUNEL method. Blue signals indicated DAPI-stained nuclei, and red signals represented cells undergoing apoptosis. Scale bar = 100 μm. (B) Quantitative analysis of fluorescence intensity of TUNEL staining. (C) Western blot detection of apoptosis-related protein expressions in spleen tissues. (D–G) Quantitative analysis of Bax, Bcl-2, Cleaved Caspase-3, and Cyt C protein expressions (mean ± SD, n = 3). (H) Safe concentration of MON in RAW264.7 cells was detected using CCK-8. (I) Cells were pretreated with MON (20 μmol/L) or ML385 (5 μmol/L) for 2 h and then administrated with LPS (1 μg/mL) for 24 h. Western blot assay for expression levels of Nrf2 and apoptosis-related proteins in RAW264.7 cells. (J–N) Quantitative analysis of Nrf2, Bax, Bcl-2, Cleaved Caspase-3, and Cyt C protein expressions (mean ± SD, n = 3). Comparisons of differences between groups were indicated by *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; ns, not significant.
In conclusion, MON alleviates sepsis-induced splenic damage by modulating immune responses and suppressing inflammation and oxidative stress. Mechanistically, MON could reduce inflammation and oxidative stress via suppression of the NF-κB/NLRP3 pathway and activation of the Nrf2 pathway and inhibit apoptosis by regulating the Bax/Cyt C/Caspase-3 signaling axis. Thus, MON may be an effective therapeutic agent for sepsis-associated spleen injury. However, this study has some limitations. For example, experiments have mainly been conducted in mouse models, and whether the results can be directly applied to humans requires further verification. In addition, the long-term safety and efficacy of MON have not yet been confirmed in clinical trials. More in-depth studies are necessary to clarify the molecular mechanisms by which MON treats sepsis, and future research should incorporate broad clinical evidence to ensure its curative efficacy.
-
All animal experiments were approved by the Institutional Animal Ethics Committee of Jiangsu Ocean University, and animal care was in accordance with the institutional guidelines.
doi: 10.3967/bes2025.008
Monotropein Alleviates Sepsis-induced Spleen Injury by Inhibiting the NF-κB/NLRP3 Axis and Activating the Nrf2 Pathway
-
Methodology, Formal analysis, and Writing-original draft: Le Bian and Feibiao Wang; Visualization and Data curation: Yue Yang; Validation and Software: Kunmei Xie; Investigation and Resources: Tingzhaoyun Hu; Methodology and Software: Jiadai Tang; Resources and Funding acquisition: Lei Wang; Supervision, Project administration, and Funding acquisition: Zibo Dong.
The authors declare that they have no conflicts of interest.
&These authors contributed equally to this work.
注释:1) Authors’ Contributions: 2) Competing Interests: -
Figure 1. Effect of MON on spleen pathological injury and inflammatory response in CLP-induced septic mice. (A) Representative images of HE staining of spleen tissues. (B) Spleen index of mice. (C–G) The mRNA levels of TNF-α, IL-1β, IL-6, iNOS and IL-10 were examined via RT-qPCR (n = 6). (H) Western blot detection of the expressions of NF-κB pathway-related proteins. (I–J) Gray-scale value analysis of p-NF-κB p65/NF-κB p65 and p-IκBα/IκBα (mean ± SD, n = 3). (K–M) Protein expressions of NLRP3 and Caspase-1 p20 were detected and quantified (mean ± SD, n = 3). Comparisons of differences between groups were indicated by *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; ns, not significant.
Figure 2. Effect of MON on oxidative stress in the spleen of CLP-induced septic mice. (A) ROS contents were detected by DHE staining. Blue signals indicated DAPI-stained nuclei and red signals indicated ROS production. (B) Quantitative analysis of ROS fluorescence intensity in spleen tissues (mean ± SD, n = 3). (C–F) Absorbance of MDA, CAT, GSH and T-AOC was measured at 532, 405, 412, and 520 nm, respectively, using a multifunctional microplate detector, and the content of each indicator was calculated (n = 6). (G–J) Protein expressions of Keap1, Nrf2 in the nucleus and Nrf2 in the cytoplasm were examined and quantified (n = 3). Comparisons of differences between groups were indicated by *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; ns, not significant.
Figure 3. Effect of MON on apoptosis in CLP-induced mice spleen tissues and LPS-treated RAW 264.7 cells. (A) Splenic cell apoptosis was detected by TUNEL method. Blue signals indicated DAPI-stained nuclei, and red signals represented cells undergoing apoptosis. Scale bar = 100 μm. (B) Quantitative analysis of fluorescence intensity of TUNEL staining. (C) Western blot detection of apoptosis-related protein expressions in spleen tissues. (D–G) Quantitative analysis of Bax, Bcl-2, Cleaved Caspase-3, and Cyt C protein expressions (mean ± SD, n = 3). (H) Safe concentration of MON in RAW264.7 cells was detected using CCK-8. (I) Cells were pretreated with MON (20 μmol/L) or ML385 (5 μmol/L) for 2 h and then administrated with LPS (1 μg/mL) for 24 h. Western blot assay for expression levels of Nrf2 and apoptosis-related proteins in RAW264.7 cells. (J–N) Quantitative analysis of Nrf2, Bax, Bcl-2, Cleaved Caspase-3, and Cyt C protein expressions (mean ± SD, n = 3). Comparisons of differences between groups were indicated by *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; ns, not significant.
-
[1] Yao RQ, Ren C, Zheng LY, et al. Advances in immune monitoring approaches for sepsis-induced immunosuppression. Front Immunol, 2022; 13, 891024. doi: 10.3389/fimmu.2022.891024 [2] Su JQ, Zhou F, Wu S, et al. Research progress on natural small-molecule compounds for the prevention and treatment of sepsis. Int J Mol Sci, 2023; 24, 12732. doi: 10.3390/ijms241612732 [3] Wu MQ, Lai HB, Peng W, et al. Monotropein: a comprehensive review of biosynthesis, physicochemical properties, pharmacokinetics, and pharmacology. Front Pharmacol, 2023; 14, 1109940. doi: 10.3389/fphar.2023.1109940 [4] Chen YE, Lu YY, Pei CY, et al. Monotropein alleviates secondary liver injury in chronic colitis by regulating TLR4/NF-κB signaling and NLRP3 inflammasome. Eur J Pharmacol, 2020; 883, 173358. doi: 10.1016/j.ejphar.2020.173358 [5] Zhang YP, Chen YE, Li BX, et al. The effect of monotropein on alleviating cisplatin-induced acute kidney injury by inhibiting oxidative damage, inflammation and apoptosis. Biomed Pharmacother, 2020; 129, 110408. doi: 10.1016/j.biopha.2020.110408 [6] Seemann S, Zohles F, Lupp A. Comprehensive comparison of three different animal models for systemic inflammation. J Biomed Sci, 2017; 24, 60. doi: 10.1186/s12929-017-0370-8 [7] Marey AM, Dkhil MA, Abdel-Moneim AE, et al. Unraveling the immune response of the spleen in sepsis using green-synthesized silver nanoparticles from pomegranate peel extracts. Microsc Res Tech, 2024; 87, 2034−42. doi: 10.1002/jemt.24575 [8] Busch K, Kny M, Huang N, et al. Inhibition of the NLRP3/IL-1β axis protects against sepsis-induced cardiomyopathy. J Cachexia Sarcopenia Muscle, 2021; 12, 1653−68. doi: 10.1002/jcsm.12763 [9] Luo J, Wang J, Zhang J, et al. Nrf2 deficiency exacerbated CLP-induced pulmonary injury and inflammation through autophagy- and NF-κB/PPARγ-mediated macrophage polarization. Cells, 2022; 11, 3927. doi: 10.3390/cells11233927 [10] Gao F, Qian MJ, Liu GY, et al. USP10 alleviates sepsis-induced acute kidney injury by regulating sirt6-mediated Nrf2/ARE signaling pathway. J Inflamm (Lond), 2021; 18, 25. doi: 10.1186/s12950-021-00291-7 -




 下载:
下载:



 Quick Links
Quick Links