-
Oxidative stress is known to be a primary culprit in nerve damage following traumatic brain injury. Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) acts as a key transcription factor in the antioxidant stress response. By binding to the downstream transcription factor heme oxygenase-1 (HO-1), Nrf2 exerts a protective effect against oxidative stress in the brain[2]. Curcumin, a low-molecular-weight polyphenolic compound derived from turmeric, possesses a broad range of pharmacological activities, including anti-inflammatory, antioxidant, lipid-lowering, antiviral, anti-infection, and anti-tumor effects. Notably, studies have shown that curcumin can activate the Nrf2/HO-1 pathway in astrocytes, thereby inhibiting oxidative stress and promoting neurological function recovery after cerebral hemorrhage[3]. At present, the effects of curcumin on the Nrf2/HO-1 signaling pathway and neuroinflammation after gas explosion in blast brain injury rats were not clarified.
Thirty male Sprague-Dawley rats (250 ± 20 g) were obtained from SPF (Beijing) Biotechnology Co., Ltd. (License key: SCXK (Jing) 2019-0010). The rats were randomly divided into three groups (n = 10/group): control (Con), model (BBI), and curcumin intervention (Cur). Animals in the Con group were neither exposed to gas explosion nor curcumin treatment. The BBI group received gas explosion treatment, and the Cur group received curcumin by gavage at a dose of 100 mg/kg every other day for seven days. For the Cur group, animals were exposed to gas explosion before being treated with curcumin. The rats in the BBI and Cur groups were anesthetized and placed in specially designed restraining cages with their heads facing the source of the explosion within sealed explosion chambers. The chambers were located 200 m from the explosion source and contained a gas mixture concentration of 9%. The specific parameters of the explosion were described previously: ignition occurred centrally, with pressures ranging between 1.6 to 2.0 kg using a fire system. Seven days after the gas explosion, rats were euthanized under anesthesia with 30% urethane. Brain tissue was collected, and blood samples were obtained from the abdominal aorta. This study was approved by the Animal Ethics Committee of Xinxiang Medical University (No. XYLL-2020007). The animal disposal method conforms to animal ethics standards.
Seven days after the explosion, gross examination revealed significant brain injury in the BBI group, characterized by cerebral congestion, cerebral edema, and visible damage (Figure 1B). This group also displayed a statistically significant increase in brain weight ratio compared to the Con group (Figure 1A and D, P < 0.05). Conversely, the Cur group showed a trend toward reduced brain weight ratio and less severe brain tissue damage (Figure 1C and D) although not statistically significant (P > 0.05). Histological examination using hematoxylin and eosin staining and transmission electron microscopy corroborated these findings. The Con group displayed normal brain tissue structure with intact neural cells, distinct nuclei, and minimal edema (Figure 1E and H). In contrast, the BBI group exhibited disrupted neural cell arrangement, loose interstitial edema, dense staining of chromatin, and apoptotic features such as dense aggregates (Figure 1F and I). The Cur group showed increased glial proliferation, relatively fewer glial cell nuclei, and cellular sparse degeneration (Figure 1G and J). The results of this study indicate that compared with the BBI group, the Cur group of rats had reduced cerebral hemorrhage, reduced brain tissue visceral ratio, and reduced pathological brain tissue damage, indicating that curcumin has a protective effect on rat brain damage caused by gas explosion blast injury. However, the mechanism of the protective effect of curcumin in the brain blast injury model is unclear.
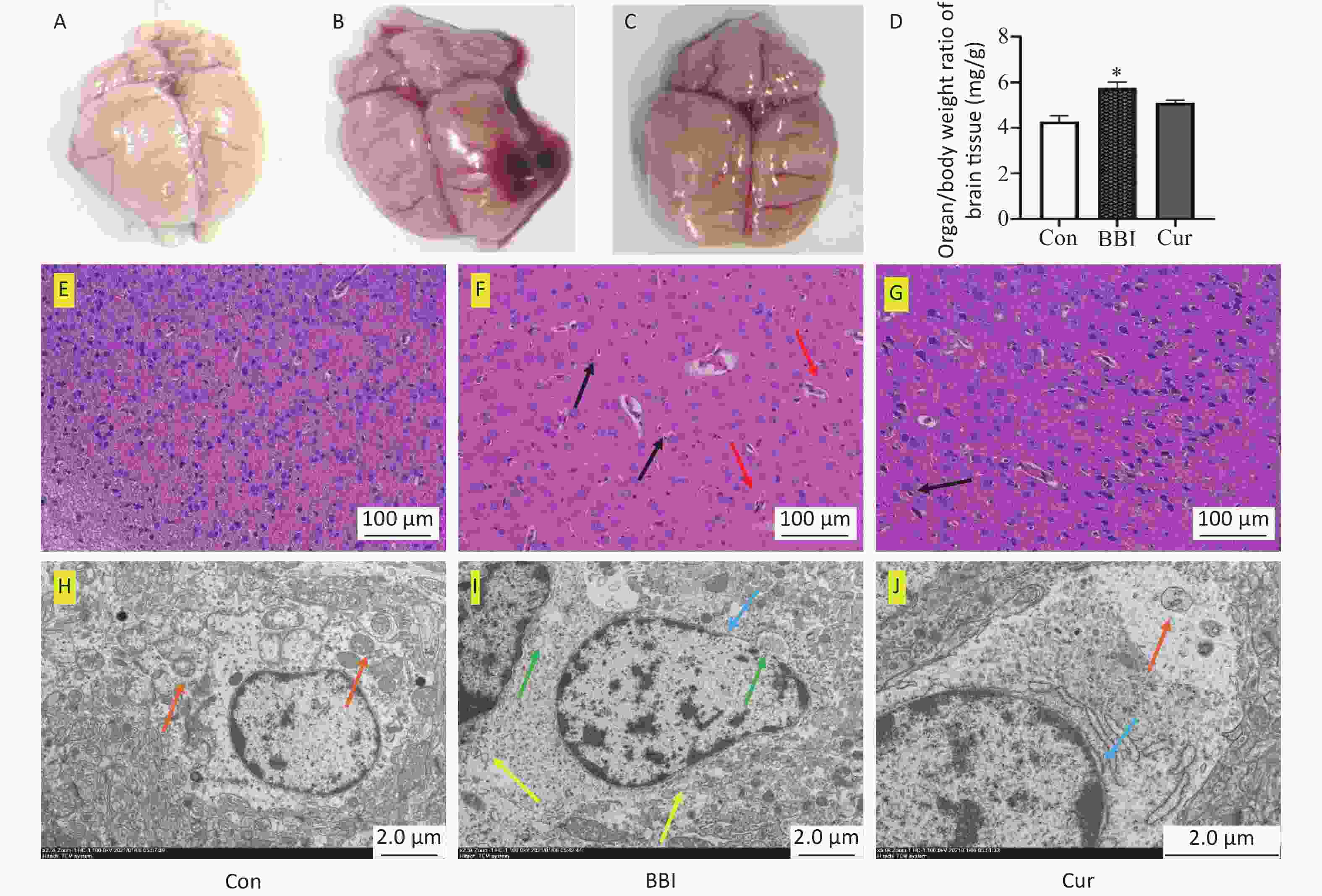
Figure 1. Effects of curcumin on gas explosion-induced brain injury in rats. Con, control group (A, E, and H); BBI, model group (B, F, and I); Cur, curcumin intervention group (C, G, and J). (A–C) Tissue appearance. (D) Brain weight ratio (means ± SD, n = 10). (E–G) Pathological Changes. (H–J) Microstructural Changes. Significant differences were observed in brain weight ratios among the experimental groups compared to the Con group (*P < 0.05). Hematoxylin and eosin staining (magnification 200 ×, scale = 100 μm): E–G; Transmission electron microscopy: H–J. Black arrows: inflammatory infiltration; Red arrows: edema and damage; Blue arrows: nuclear membrane expansion; Yellow arrows: endoplasmic reticulum expansion, vesicles; Green arrows: swelling and vacuolization of mitochondria; Brown arrows: relatively intact mitochondrial structure. Standard deviations are denoted by error bars on graphs. SD, standard deviation.
Studies have found that in the traumatic brain cortical injury animal model, curcumin can reduce traumatic brain injury by lowering the levels of interleukin (IL)-1β and IL-6, promoting microglial cell M2 polarization and reducing complement 1q-like-3 protein (C1ql3) expression[4]. The latest in vitro and in vivo studies have shown that curcumin significantly inhibits microglial pyroptosis and pro-inflammatory responses by inhibiting the nuclear factor kappa B (NF-κB)/NLRP3 signaling pathway, alleviating stroke-induced white matter injury, and improving functional outcomes[5]. Additionally, curcumin significantly reduced the serum levels of inflammatory factors IL-6, IL-1β, NF-κB, and tumor necrosis factor-alpha (Figure 2A–D), and the protein expression levels of IL-6 and NF-κB (Supplementary Figure S1, available in www.besjournal.com) in rats’ brains after a gas explosion, suggesting that curcumin may alleviate brain nerve damage by mitigating the inflammatory response after brain blast injury.
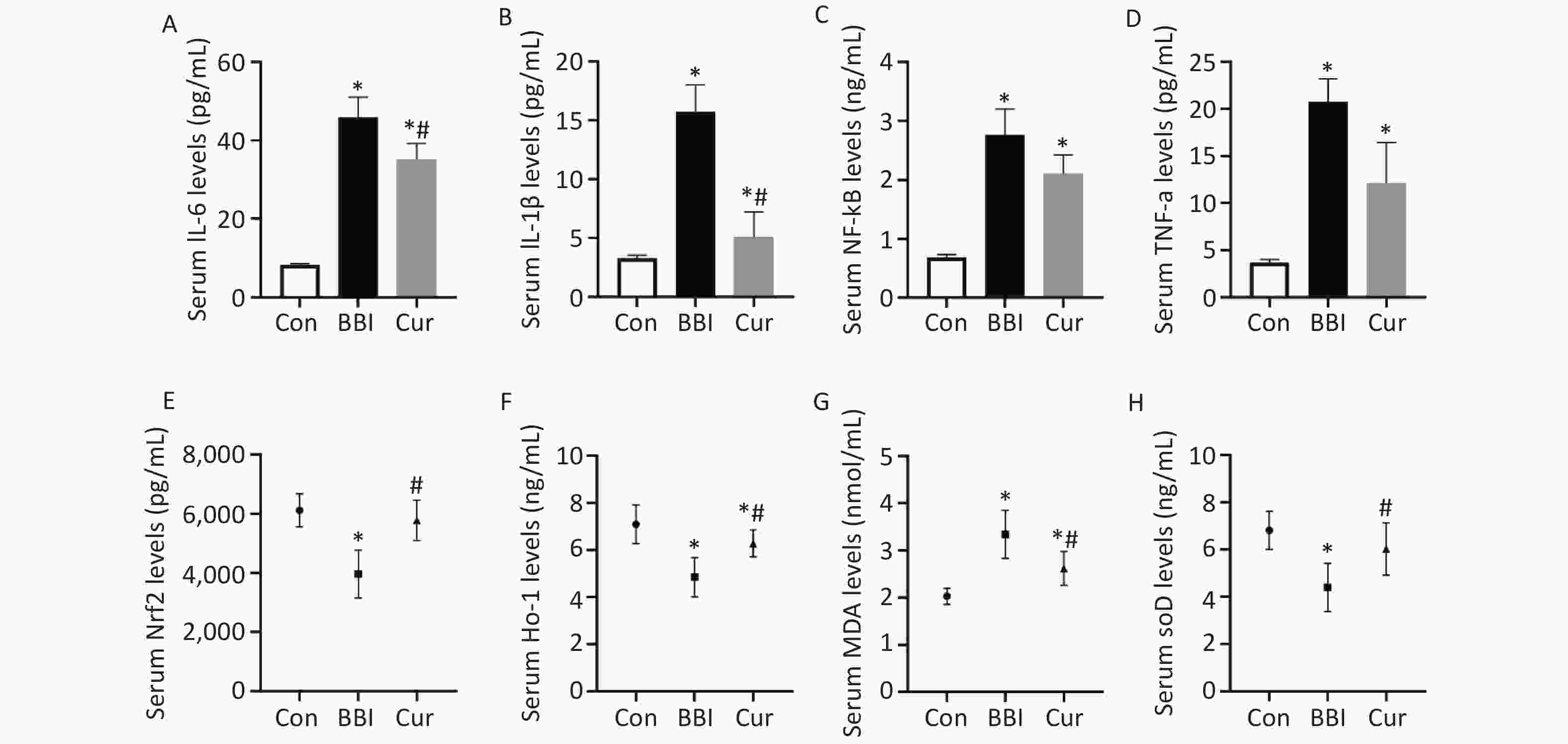
Figure 2. Effects of curcumin on the neuroinflammatory factors (A–D), Nrf2/HO-1 pathway factor (E and F), oxidative stress (G and H), and levels induced by gas explosion in rats’ serum of various experimental groups (means ± SD, n = 10). Con, control group; BBI, model group; Cur, curcumin intervention group. *P < 0.05, compared to the Con group; #P < 0.05, compared to the BBI group. Standard deviations are denoted by error bars on graphs. SD, standard deviation.
The precise molecular mechanisms underlying curcumin’s anti-inflammatory and neuroprotective effects remain unclear. However, research suggests that Nrf2 activation plays a crucial role by increasing the body’s antioxidant capacity and protective proteins such as brain-derived neurotrophic factor, HO-1, anti-apoptotic Bcl-2 molecule, and the anti-inflammatory IL-10. Additionally, Nrf2 activation can interact with the inflammatory transcription factor NF-κB, exerting anti-inflammatory effects. NF-κB is a key regulator of inflammation and can also inhibit the transcription of genes downstream of Nrf2[6]. The observed decrease in NF-κB levels in the Cur group further supports the notion that Nrf2 has a dual role in protecting the central nervous system and reducing inflammation (Supplementary Figure S1).
The results of this study suggest that curcumin’s brain-protective effect may be linked to the activation of the Nrf2/HO-1 signaling pathway. Consistent with previous findings demonstrating curcumin’s ability to regulate Nrf2 in protecting the brain from oxidative stress and inflammation[7], this experiment found significantly lower Nrf2 and HO-1 expression levels in the brains of BBI group rates compared to the Con group after seven days of gas explosion. Conversely, the Cur group displayed significantly higher expression levels of Nrf2 and HO-1 (Figure 2E and F, Figure 3A and B) compared to the BBI group, although IL-10 levels remained unchanged (Figure 3F). Furthermore, HO-1, a downstream signaling molecule of Nrf2, possesses significant brain-protective and antioxidant stress-reducing effects. Under physiological conditions, Nrf2 binds to Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm. When exogenous inducers enter the body, they trigger Nrf2 to dissociate from Keap1, enter the nucleus, bind to antioxidant response elements, and regulate the transcription and translation of HO-1. This initiates HO-1’s antioxidant and anti-inflammatory effects, ultimately reducing the production of inflammatory mediators[8].
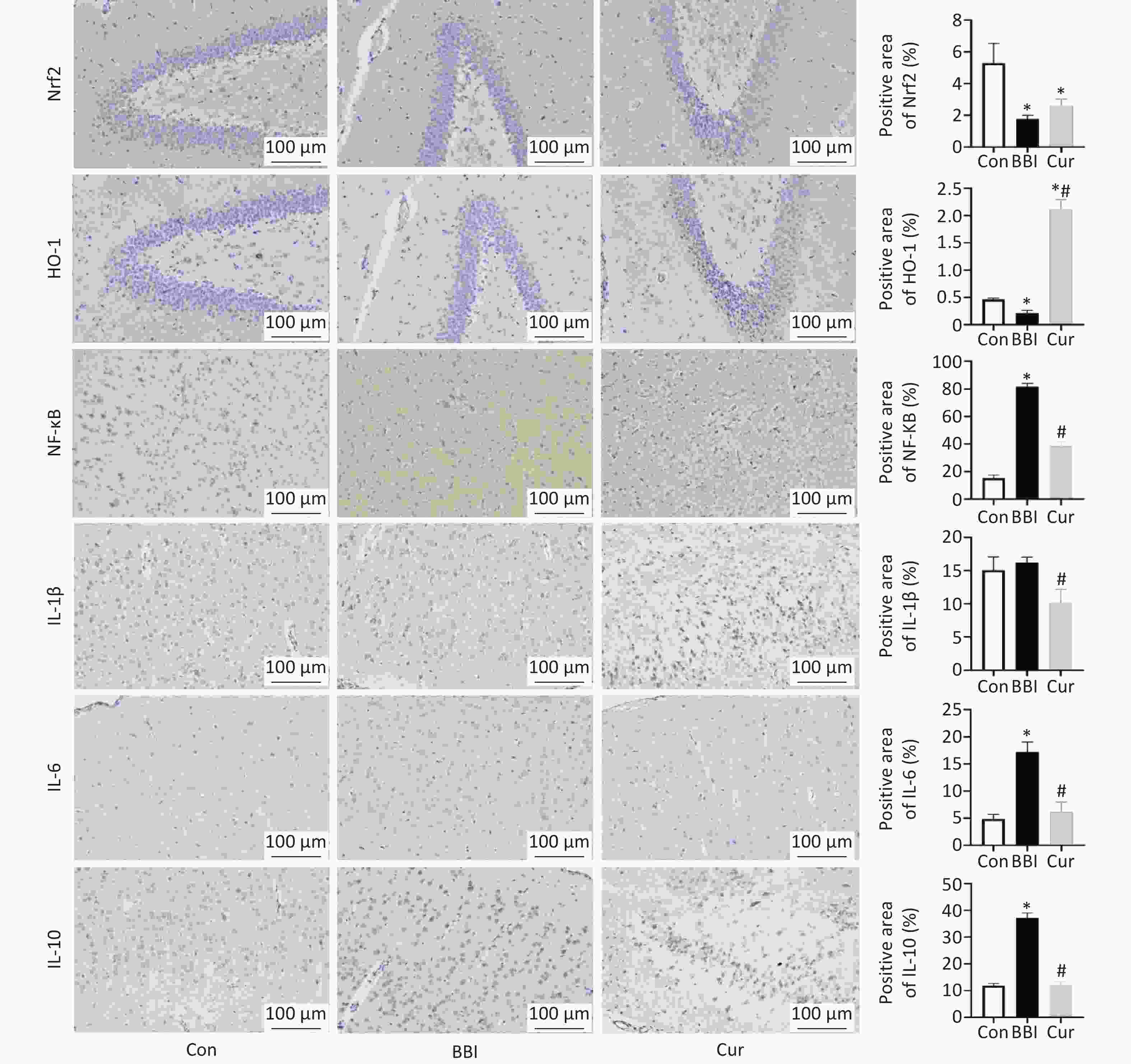
Figure 3. Effects of curcumin on the expression and distribution levels of Nrf2/HO-1 pathway (A and B) and neuroinflammatory factor (C–F) proteins induced by gas explosion in the rats’ brains of various experimental groups (means ± SD, n = 3). Con, Control group; BBI, Model group; Cur, Curcumin intervention group. *P < 0.05, compared to the Con group; #P < 0.05, compared to the BBI group. Standard deviations are denoted by error bars on graphs. SD, standard deviation.
To further elucidate the molecular mechanism by which curcumin mediates inflammation and oxidative stress, we employed immunohistochemistry to assess the effects of curcumin on Nrf2 and HO-1 expression and distribution in brain tissues. The results demonstrated that gas explosions significantly reduced the expression of Nrf2 and HO-1, while curcumin intervention reversed this process. This suggests that curcumin may exert brain nerve injury protection by promoting the expression of Nrf2 and HO-1 (Figure 3A and B). Similar findings from other studies have shown that curcumin can protect against focal ischemic brain injury in rats by upregulating the Nrf2 and HO-1 transcription factors[9]. These results collectively indicate that curcumin’s anti-neuroinflammatory effects may be linked to the activation of the Nrf2/HO-1 signaling pathway. However, further exploration is necessary to fully understand the specific molecular mechanisms involved.
Previous studies have shown that curcumin protects against cerebral hemorrhage by activating the Nrf2/HO-1 pathway, inhibiting oxidative stress, and reducing inflammation[3]. This study aligns with those findings, demonstrating that curcumin treatment in the gas explosion model increased Nrf2 and HO-1 protein expression, inhibited inflammatory factors (IL-6, IL-1β, and NF-κB), and reduced oxidative stress markers (malondialdehyde) while increasing antioxidant defenses (superoxide dismutase) (Figure 2C–H, Figure 3C–E). These observations suggest that curcumin’s protective effects in this model may be mediated by activating the Nrf2/HO-1 pathway and reducing inflammation and oxidative stress. Further research suggests that curcumin’s neuroprotective properties may involve correcting calcium imbalances in microglial cells, improving mitochondrial function and energy metabolic pathways, and inhibiting P2X7 receptor activation[10]. Additionally, curcumin may promote M2 polarization of astrocytes and modulate monophosphate-activated protein kinase signaling pathways, potentially contributing to improved neurological function[4].
In conclusion, curcumin treatment following a gas explosion appears to alleviate neuroinflammation, reduce oxidative stress, and improve neurological outcomes, possibly through the activation of the Nrf2/HO-1 signaling pathway. Further investigation into these and other potential mechanisms is warranted to establish a strong theoretical foundation for the clinical application of curcumin in treating brain injuries caused by coal mine gas explosions.
doi: 10.3967/bes2024.116
Effects of Curcumin on Neuroinflammation and the Nrf2/HO-1 Pathway in Rat Brains Following Gas Explosion
-
Abstract: Gas explosions, a major occupational hazard in China’s coal industry, endanger the lives and health of miners. These explosions cause a specific type of traumatic brain injury with complex mechanisms, leading to disability and death. A study by Zhao et al. using magnetic resonance imaging on 49 gas explosion survivors revealed significant damage to brain regions like the hippocampus and cerebral cortex. Similarly, our recent animal experiments conducted in a real tunnel environment demonstrated that gas explosions alter rat neurobehavior, cause microscopic structural damage to brain tissue, and impaired function[Xinwen Dong conceived and designed the experiments, analyzed the data and wrote the manuscript. Yaguang Su, Zheng Luo, Lyufei Deng, Xiaofeng Han, Yifang Liang, and Yichun Bai conceived, designed, and performed the experiments. Sanqiao Yao and Weidong Wu analyzed the data and wrote the manuscript. Jia Cao and Linqiang Tian provided experimental materials and sites. Wenjie Ren reviewed and edited the manuscript. All authors read and approved the final manuscript.
1 ]. However, the underlying physiological and pathological mechanisms of this blast-induced brain injury remain unclear.
The authors declare no conflict of interest.
注释:1) Authors’ Contributions: 2) Authors’ Contributions: 3) Conflicts of Interest: -
Figure 1. Effects of curcumin on gas explosion-induced brain injury in rats. Con, control group (A, E, and H); BBI, model group (B, F, and I); Cur, curcumin intervention group (C, G, and J). (A–C) Tissue appearance. (D) Brain weight ratio (means ± SD, n = 10). (E–G) Pathological Changes. (H–J) Microstructural Changes. Significant differences were observed in brain weight ratios among the experimental groups compared to the Con group (*P < 0.05). Hematoxylin and eosin staining (magnification 200 ×, scale = 100 μm): E–G; Transmission electron microscopy: H–J. Black arrows: inflammatory infiltration; Red arrows: edema and damage; Blue arrows: nuclear membrane expansion; Yellow arrows: endoplasmic reticulum expansion, vesicles; Green arrows: swelling and vacuolization of mitochondria; Brown arrows: relatively intact mitochondrial structure. Standard deviations are denoted by error bars on graphs. SD, standard deviation.
Figure 2. Effects of curcumin on the neuroinflammatory factors (A–D), Nrf2/HO-1 pathway factor (E and F), oxidative stress (G and H), and levels induced by gas explosion in rats’ serum of various experimental groups (means ± SD, n = 10). Con, control group; BBI, model group; Cur, curcumin intervention group. *P < 0.05, compared to the Con group; #P < 0.05, compared to the BBI group. Standard deviations are denoted by error bars on graphs. SD, standard deviation.
Figure 3. Effects of curcumin on the expression and distribution levels of Nrf2/HO-1 pathway (A and B) and neuroinflammatory factor (C–F) proteins induced by gas explosion in the rats’ brains of various experimental groups (means ± SD, n = 3). Con, Control group; BBI, Model group; Cur, Curcumin intervention group. *P < 0.05, compared to the Con group; #P < 0.05, compared to the BBI group. Standard deviations are denoted by error bars on graphs. SD, standard deviation.
-
[1] Dong XW, Yao SQ, Wu WD, et al. Short-term effect of gas explosion in real roadway environment on rats' brain neural behavior. J Hyg Res, 2020; 49, 889−94. (In Chinese [2] AbdElrazek DA, Ibrahim MA, Hassan NH, et al. Neuroprotective effect of quercetin and nano-quercetin against cyclophosphamide-induced oxidative stress in the rat brain: Role of Nrf2/ HO-1/Keap-1 signaling pathway. Neurotoxicology, 2023; 98, 16−28. doi: 10.1016/j.neuro.2023.06.008 [3] Duan CY, Wang HB, Jiao D, et al. Curcumin restrains oxidative stress of after intracerebral hemorrhage in rat by activating the Nrf2/HO-1 pathway. Front Pharmacol, 2022; 13, 889226. doi: 10.3389/fphar.2022.889226 [4] Zhang M, Hao ZL, Wu JY, et al. Curcumin ameliorates traumatic brain injury via C1ql3-mediated microglia M2 polarization. Tissue Cell, 2023; 84, 102164. doi: 10.1016/j.tice.2023.102164 [5] Ran YY, Su W, Gao FH, et al. Curcumin ameliorates white matter injury after ischemic stroke by inhibiting microglia/macrophage pyroptosis through NF-κB suppression and NLRP3 inflammasome inhibition. Oxid Med Cell Longev, 2021; 2021, 1552127. [6] Buendia I, Michalska P, Navarro E, et al. Nrf2-ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther, 2016; 157, 84−104. doi: 10.1016/j.pharmthera.2015.11.003 [7] Patel H, McIntire J, Ryan S, et al. Anti-inflammatory effects of astroglial α7 nicotinic acetylcholine receptors are mediated by inhibition of the NF-κB pathway and activation of the Nrf2 pathway. J Neuroinflammation, 2017; 14, 192. doi: 10.1186/s12974-017-0967-6 [8] Shahcheraghi SH, Salemi F, Peirovi N, et al. Nrf2 regulation by curcumin: molecular aspects for therapeutic prospects. Molecules, 2021; 27, 167. doi: 10.3390/molecules27010167 [9] Yang CH, Zhang XJ, Fan HG, et al. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res, 2009; 1282, 133−41. doi: 10.1016/j.brainres.2009.05.009 [10] Feng LQ, Luo GD, Li YH, et al. Curcumin-dependent phenotypic transformation of microglia mediates resistance to pseudorabies-induced encephalitis. Vet Res, 2023; 54, 25. doi: 10.1186/s13567-023-01149-x -
 24193+Supplementary Materials.pdf
24193+Supplementary Materials.pdf

-




 下载:
下载:






 Quick Links
Quick Links