-
Exposure to free silica induces silicosis and myofibroblasts are regarded as primary effector cells. Fibrocytes can differentiate into myofibroblast. Therefore, the present study was designed to investigate whether fibrocytes participate in silicosis. The rat model of silicosis was established. Hematoxylin-eosin stainings and Masson stainings were used to evaluate the histopathology and collagen deposition. Flow cytometry and immunofluorescence were performed to detect the number of fibrocytes and their contribution to myofibroblasts. Results showed that fibrocytes participate in silicosis. Trend analysis of different sources of myofibroblasts during silicosis indicated that fibrocytes and lung type Ⅱ epithelial cell-derived myofibroblasts play an important role in the early stage of silicosis, while resident lung fibroblast-derived myofibroblasts play a predominant role during the fibrosis formative period.
Chronic inhalation of crystalline silica (SiO2) is associated with the development of silicosis, which is characterized by tissue remodelling, fibroproliferation, and deposition of extracellular matrix (ECM). Myofibroblasts, as key effector cells, are believed to play a major role in the pathogenesis of fibrotic diseases. The source of these cells has been intensively debated. Resident lung fibroblasts were conventionally thought to be the main source of myofibroblasts. However, recent studies have demonstrated that epithelial-mesenchymal differentiation or recruitment of fibrocytes also contribute to myofibroblasts in lung fibrosis. Little attention has been focused on fibrocytes and the contribution of different sources of myofibroblasts in silicosis.
Among the possible sources of lung myofibroblasts, fibrocytes are bone marrow-derived cells that co-express CD45 and collagen type Ⅰ (CD45+ Col Ⅰ+)[1]. Fibrocytes participate in both tissue fibrosis and tissue remodelling by producing ECM and matrix metalloproteinases[2]. Recent studies have reported that the number of fibrocytes is obviously increased in patients with idiopathic pulmonary fibrosis (IPF) and that higher numbers of them are related to early mortality[3]. Taken together, a growing body of evidence indicates that fibrocytes participate in lung. In our present study, we sought to evaluate the contribution of fibrocytes to silicosis. We compared lung tissue and peripheral blood from rats with silicosis and control rats for the presence and number of fibrocytes and contribution of the three sources of myofibroblasts during the process of experimental silicosis. Our findings support the notion that fibrocytes may participate in silicosis and that different sources of myofibroblasts may play roles at different stages of silicosis.
The animal experiments were approved by the Experimental Animal Ethics Committee of Zhengzhou University, and our study was conducted in accordance with the guidelines of the Chinese Association of Laboratory Animal Care. The SD rats were randomly divided into the model group and control group (n = 36 per group). The rats in the model group were administered a single intratracheal instillation of SiO2 solution (100 mg/0.5 mL per rat) and those of control group were instilled an equal amount of 0.9% saline. All of the rats were anaesthetized with chloral hydrate and then sacrificed by cervical dislocation for further study at 1, 2, 3, 6, 9, and 12 weeks (n = 12 per week) after silica/saline instillation.
Hematoxylin-eosin (HE) and Masson stainings were used to evaluate the histopathology and collagen deposition. The extent of lung fibrosis was evaluated by the measurement of the area of fibrosis (%) using Image-Pro Plus 6.0 software (Media Cybernetics, MD, USA). Double-colour immunofluorescence was performed to identify fibrocytes or different sources of myofibroblasts in the lung tissue. The fibrocytes were labelled by CD45+ Collagen Ⅰ+. The fibrocytes-derived myofibroblasts were labelled by CD45+ α-smooth muscle actin+ (SMA). The lung type Ⅱ epithelial cells (AT Ⅱ)-derived myofibroblasts were labelled by human pulmonary surfactant associatedprotein (SPC+ α-SMA+). The nuclei was stained by 4', 6'-diamidino-2-phenylindole dihydrochloride (DAPI). The sections were observed by a Nikon Eclipse Ti microscope (Nikon, Totyo, Japan). For the counting of fibrocyte-derived collagen Ⅰ-secreting cells or fibrocyte-derived and AT Ⅱ-derived myofibroblasts in the lungs, two observers assessed the number of double-positive cells. Ten fields were randomly selected to be examined, and the numbers of double positive cells were counted and calculated as the mean number of positively stained cells per total number of collagen Ⅰ or α-SMA positive cells at sections of the lungs at 200× magnification.
The statistical analysis was performed using SPSS 21.0 (IBM, NC, USA). Comparisons were evaluated by Student's t test. P values that were less than 0.05 were used to define statistically significant differences.
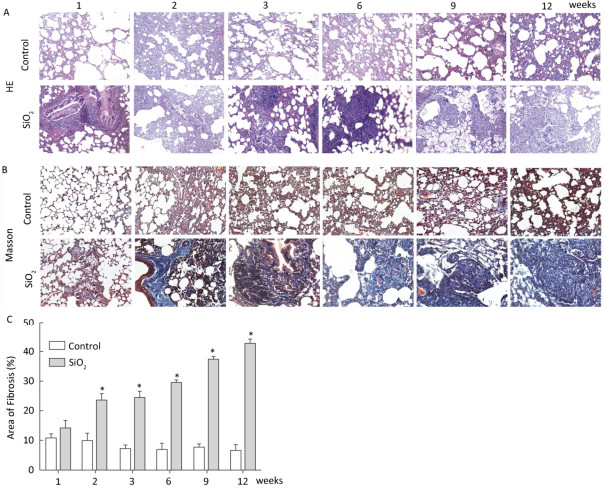
In the present study, the rat silicosis model was successfully established (Figure S1 available in www.besjournal.com).
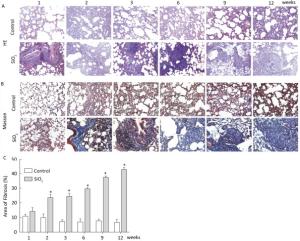
Figure Figure S1. SiO2 exposure induces lung inflammation and fibrosis. Pathological changes and morphological alteration of lung tissues were evaluated by HE staining (A, magnification × 200). Masson trichrome staining was used to determine collagen deposition in lung. Areas of fibrosis (%) were quantified with Image-Pro Plus 6.0 software. The areas of fibrosis was increased from week 2 to week 9 (B and C, magnification ×200). Data are expressed as mean ± SD. *P < 0.05, vs. control.
Fibrocytes comprise approximately 0.1% to 1% of human peripheral blood leukocytes in healthy individuals[4]. The number of fibrocytes increases and they rapidly enter sites of tissue injury once the skin is injured. Similar results were found in this study. Fibrocytes exist and are increased in the peripheral blood early during the inflammatory response (at 2 and 3 weeks) after SiO2 exposure (Figure 1A and 1C). The results of our study suggest that SiO2 could promote fibrocyte differentiation both in vitro and in vivo and that the increased number of fibrocytes can be observed in the early stage of silicosis in the peripheral blood[5]. Flow cytometry (FCM) analysis showed that the percent of fibrocytes in the lung tissue was obviously increased after SiO2 exposure from 1 to 12 weeks (Figure 1B and 1D). At 1 week, the number of fibrocytes weeks was increase in lung while in blood there is no obvious change. We assume that fibrocytes can migrate rapidly to lung tissue to participate in injury repair and that the body can still maintain homeostasis at 1 week. However, the lung microenvironment is in disorder with the prolongation of SiO2 exposure, as more fibroblast-like cells need to participate in pathological repair and reconstruction. At this time, bone marrow may supply more fibrocyte precursor cells to maintain a high level of fibrocytes in the peripheral blood or lung tissue. The further study confirmed CD45+ collagen Ⅰ+ fibrocytes were existent in the lung tissue. CD45+ collagen Ⅰ+ fibrocytes contributed to approximately 10% to 16% of the collagen Ⅰ-expressing cells in the lungs of SiO2-exposed rats which suggests that fibrocytes play a role in the ECM deposit in silicosis (Figure 2A and 2B).
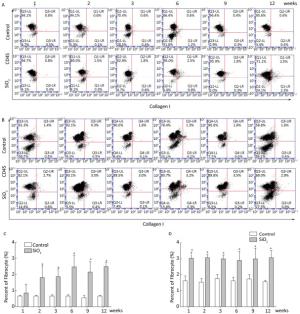
Figure 1. SiO2 exposure increased the number of fibrocytes in peripheral blood and lung tissue. The number of fibrocytes in peripheral blood was increased from week 1 to week 9, there were no significant difference was observed at week 1 (A and C). The number of fibrocytes in lung tissue was increased from week 1 to week 12 (B and D). Data are expressed as mean ± SD. *P < 0.05 vs. control.
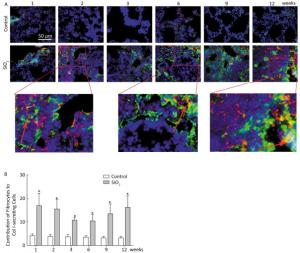
Figure 2. Fibrocyte is exist in lung tissue (A) and SiO2 exposure increased the percent of fibrocyte derived collagen Ⅰ secreting cells (B). The red represents CD45 and green represents Collagen Ⅰ. The arrow points to Fibrocytes (yellow). Data are expressed as mean ± SD. *P < 0.05 vs. control.
Based on animal models of fibrosis in parenchymal organs, fibrocytes accounted for approximately 5% to 20% of myofibroblasts in fibrotic organs. The highest amounts were in bleomycin-induced lung fibrosis, of which fibrocyte-derived myofibroblasts comprise 25% to 50% of the total number of myofibroblasts[6]. These data suggest that fibrocyte recruitment may contribute to lung fibrosis. Silicosis is a type of fibrotic disease, and the number of fibrocytes increases during the process of silicosis. Therefore, we wonder whether there are fibrocyte-derived myofibroblasts, what is their contribution, and what is the interaction of the different sources of myofibroblasts in the course of silicosis. To work out the above questions, CD45+ α-SMA+ and SPC+ α-SMA+ were used to identified fibrocyte-derived myofibroblasts and AT Ⅱ-derived myofibroblasts, respectively. The results showed that fibrocyte-derived myofibroblasts (15%-35%, Figure 3A and 3C) and AT Ⅱ-derived myofibroblasts (9%-21%, Figure 3B and 3D) were increased in the lung tissue of silicosis rats at different time points. We calculated the contribution of resident fibroblast-derived myofibroblasts as being approximately 40% to 65% according to the other two sources of myofibroblast contributions. As showed in Figure 3E, the numbers of fibrocyte-derived and AT Ⅱ-derived myofibroblasts have increasing trends at the inflammatory response period (at 1, 2, and 3 weeks) and then decreasing trends at the fibrotic period (at 6, 9, and 12 weeks). However, those of resident fibroblast-derived myofibroblasts in the lungs showed opposite trends. The dynamic changes of different sources of myofibroblasts indicated that they may play roles in different time points of silicosis. Fibrocyte-derived and AT Ⅱ-derived myofibroblasts may act mainly during the inflammatory response period. Fibrocytes are well-known to be capable of inflammatory chemotaxis and secreting chemokines, including inflammatory factors and chemokines, which not only maintain the local inflammatory microenvironment but also recruit many types of cells to amplify the inflammatory response[7]. We assume that fibrocytes may maintain the inflammatory microenvironment through pro-inflammatory and paracrine functions in the early stage of silicosis and may initiate the later stage of the fibrotic process. Resident fibroblast- derived myofibroblasts in the lungs play roles in the pathological fibrotic process in the late stage of silicosis. The cells in the lungs released a large number of cytokines that may promote lung resident fibroblasts to differentiate into myofibroblasts, and these myofibroblasts are capable of a greater proliferation and secretion of collagens. It is remarkable that fibrocytes can lose their CD45 phenotype in vitro[8]. Perhaps this phenomenon exists in vivo. With an increased SiO2 exposure time, fibrocytes migrated to the lungs and lost their CD45 phenotype. According to our current calculation method, these cells are counted into resident fibroblasts. Thus, using the CD45+ α-SMA+ method to identify fibrocyte-derived myofibroblasts may underestimate its contributions and overestimate the contribution of lung resident fibroblasts.
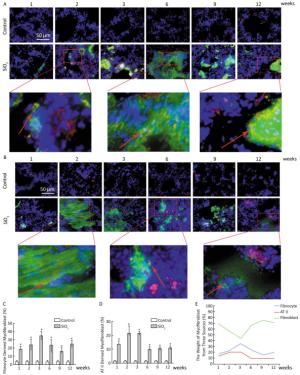
Figure 3. The contribution of different sources of myofibrobalsts. The red represents CD45 and green represents α-SMA and the number of fibrocytes-derived myofibroblasts (yellow) was increased from week 1 to week 12 (A and C). The red represents SPC and green represents α-SMA and the number of AT Ⅱ-derived myofibroblasts (yellow) was elevated from week 1 to week 12 (B and D). The trend changing analysis of different sources of myofibrobalsts (E). Data are expressed as mean ± SD. *P < 0.05 vs. control.
Our study suggests that fibrocytes may participate in silicosis and play an important role in the early stage of silicosis. In addition, it is feasible to block migration of fibrocytes due to their existence in peripheral blood, so fibrocytes may represent a novel therapeutic target for developing more effective treatments against silicosis.
The authors of the present study are grateful to the staff of the college of Public Health of the Zhengzhou University.
No conflict of interests to declare.
doi: 10.3967/bes2018.040
-
-
Figure S1. SiO2 exposure induces lung inflammation and fibrosis. Pathological changes and morphological alteration of lung tissues were evaluated by HE staining (A, magnification × 200). Masson trichrome staining was used to determine collagen deposition in lung. Areas of fibrosis (%) were quantified with Image-Pro Plus 6.0 software. The areas of fibrosis was increased from week 2 to week 9 (B and C, magnification ×200). Data are expressed as mean ± SD. *P < 0.05, vs. control.
Figure 1. SiO2 exposure increased the number of fibrocytes in peripheral blood and lung tissue. The number of fibrocytes in peripheral blood was increased from week 1 to week 9, there were no significant difference was observed at week 1 (A and C). The number of fibrocytes in lung tissue was increased from week 1 to week 12 (B and D). Data are expressed as mean ± SD. *P < 0.05 vs. control.
Figure 3. The contribution of different sources of myofibrobalsts. The red represents CD45 and green represents α-SMA and the number of fibrocytes-derived myofibroblasts (yellow) was increased from week 1 to week 12 (A and C). The red represents SPC and green represents α-SMA and the number of AT Ⅱ-derived myofibroblasts (yellow) was elevated from week 1 to week 12 (B and D). The trend changing analysis of different sources of myofibrobalsts (E). Data are expressed as mean ± SD. *P < 0.05 vs. control.
-
[1] Reese C, Lee R, Bonner M, et al. Fibrocytes in the fibrotic lung: altered phenotype detected by flow cytometry. Front Pharmacol, 2014; 5, 141. doi: 10.3389/fphar.2014.00141/full [2] Medina A, Ghahary A. Reprogrammed fibrocytes induce a mixed Th1/Th2 cytokine response of naive CD4(+) T cells. Mol Cell Biochem, 2011; 346, 89-94. doi: 10.1007/s11010-010-0595-2 [3] Moeller A, Gilpin SE, Ask K, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med, 2009; 179, 588-94. doi: 10.1164/rccm.200810-1534OC [4] Bucala R, Spiegel LA, Chesney J, et al. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med, 1994; 1, 71-81. https://www.researchgate.net/publication/14411730_Circulating_Fibrocytes_Define_a_New_Leukocyte_Subpopulation_That_Mediates_Tissue_Repair [5] Li J, Yao W, Hou JY, et al. Crystalline Silica Promotes Rat Fibrocyte Differentiation in Vitro, and Fibrocytes Participate in Silicosis in Vivo. Biomed Environ Sci, 2017; 30, 649-60. http://www.besjournal.com/Articles/Archive/2017/No9/201710/t20171011_154081.html [6] Gomperts BN, Strieter RM. Fibrocytes in lung disease. J Leukoc Biol, 2007; 82, 449-56. doi: 10.1189/jlb.0906587 [7] Garcia de Alba C, Buendia-Roldan I, Salgado A, et al. Fibrocytes contribute to inflammation and fibrosis in chronic hypersensitivity pneumonitis through paracrine effects. Am J Respir Crit Care Med, 2015; 191, 427-36. doi: 10.1164/rccm.201407-1334OC [8] Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest, 2004; 114, 438-46. doi: 10.1172/JCI200420997 -




 下载:
下载:



 Quick Links
Quick Links