-
Intestinal ischemia/reperfusion (II/R) is a severe clinical circumstance which is frequently apparent in situations including midgut volvulus, superior mesenteric vessels closure and hemorrhagic shock. II/R not only brings about severe intestinal local damage but also leads to remote organs injury including lung, liver etc[1]. Lung injury is the most common and severe complications which manifested as pulmonary susceptibility, respiratory dysfunction, and even acute respiratory distress syndrome (ARDS)[2].
Recent studies have indicated that coronavirus disease 2019 (COVID-19) associated with intestinal hypoperfusion may further deteriorate barrier dysfunction and acute lung injury (ALI) via positive feedback mechanism[3]. However, the clinical treatment of II/R-induced lung injury is still challenging, because of an incomplete understanding of its molecular mechanism. Thus, identify the mechanism and relevant therapeutic agent is important.
Reactive oxygen species (ROS) exacerbates the capillaries disrupture and induced endothelial and epithelial lesion by eliciting cytokines in lung injury[2,4]. Moreover, macrophage polarization is involved. M2 subtype macrophages exhibits anti-inflammation and promote tissue repair roles while M1 macrophages exacerbate inflammatory reaction with pathogen-killing abilities[4].
TIPE2, tumor necrosis factor-α-induced protein 8 (TNFAIP8)-like 2 (TNFAI P8L2), is required for negative regulation of inflammatory and innate immune responses[5]. DU Y, et al. reported TIPE2 alleviates inflammatory cascade via nuclear factor kappa B (NF-κB). Moreover, TIPE2 inhibits apoptosis and oxidative burst via nicotinamide adenine dinucleotide phosphate oxidase 2 (Nox-2) in cerebral ischemia/reperfusion[6]. Kong Q, et al. demonstrated therapeutic potential of TIPE2 in ALI challenged by myocardial ischemia[7]. No report exerts the role of TIPE2 in II/R- induced ALI.
Apocynin is isolated due to the immune-regulation activity compound of rhizome from P. kurroa, a native plant derived and distribute to mountains of Nepal, Pakistan and China. Pintard C et.al. reported apocynin is a promising agent for asthma, arthritis and cardio-vascular diseases via anti-oxidative and anti-inflammatory independent of Nox-2[8]. In addition, apocynin exhibits protective role in stroke resembles TIPE2 agonist[6]. Herein, we explored the role of apocynin in II/R-induced ALI and the role of TIPE2.
Male C57BL/6J mice weighing of 18–22 g were purchased from Dalian Medical University Animal Experimental Center (No. SYXK 2018-0007). All series of steps were executed according to the institutional animal care guiding principles and certified by the Institutional Ethics Committee.
Mice II/R model were built. Briefly, superior mesenteric artery (SMA) was isolated after laparotomy and blocked softly with a non-invasive microvascular clip for 45 minutes. Then clips were removed after 240 minutes. Sham animals only accepted laparotomy. To test the effect of apocynin on II/R-induced ALI, animals were randomized divided into 3 groups (n = 8): (1) Sham group, (2) II/R group, (3) Apocynin (II/R+apocynin) group. In group (3), mice were pretreated with apocynin (4.8 mg/kg) intraperitoneally for 3 consecutive days. Animals in group (2) and group (1) were accepted equal volume of vehicle dimethylsulfoxide (DMSO, 1%).
To evaluate whether TIPE2 is required during protection of apocynin on II/R- induced ALI, II/R mice were randomized divided into 2 groups (n = 6): (1) II/R+TIPE2-siRNA group, (2) II/R+TIPE2-siNC group, then all animals suffered II/R and pretreated with apocynin. After reperfusion, all mice were collected blood samples from the abdominal aorta and sacrificed consequently.
For histological preparations, separated lung and intestinal tissues were washed using phosphate buffered saline (PBS) and fixed in 4% paraformaldehyde overnight. Followed mechanized dewatering via a ranked-alcohol instruments, tissues were re-implanted in paraformaldehyde, sectioning at 5 microns and dyeing with hematoxylin-eosin (H&E). Scores of lungs and intestinal pathology were evaluated.
Monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β) were determined using mice enzyme-linked immunosorbent assay (ELISA) kits (BOSTER Bioengineering Co Ltd, Wuhan, China). In addition, lung wet/dry (W/D) ratio was determined, separated lung tissue was weighed immediately after resection to obtain the wet weight, and then transferred to 80 °C oven for 24 hours drying, and weighed again to obtain dry weight. Bronchoalveolar lavage fluid protein (BALFP) was gathered according to the procedure of Cox (Cox, 1996) and centrifuged (1,000 rpm × 10 min). Protein from supernatant was determined by the Reagent assay kit. Malondialdehyde (MDA) was determined according to the manufacturer’s instructions (Nanjing Jiancheng Ltd., Nanjing, China).
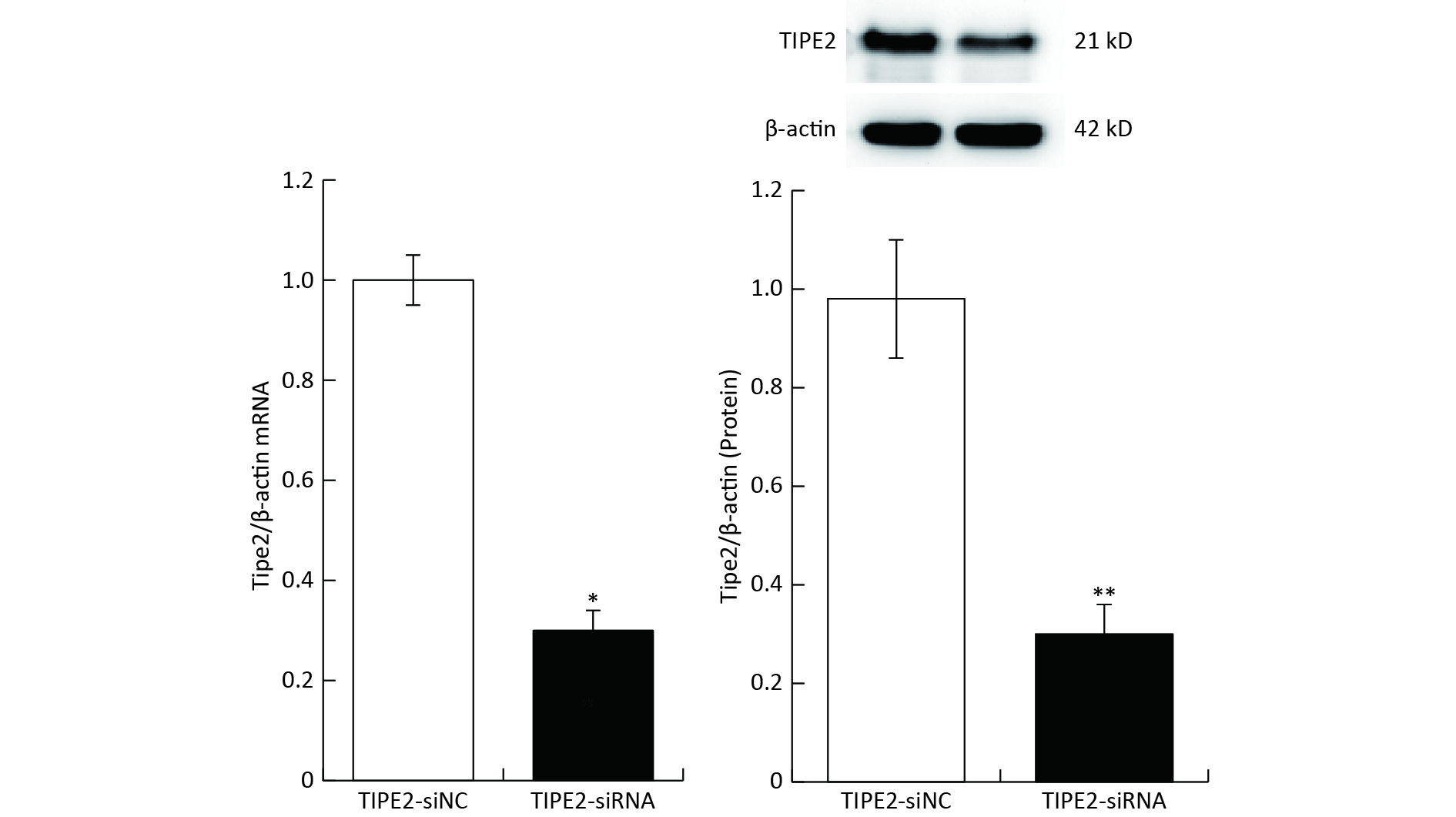
The carboxy-fluorescein (FAM) labeled TIPE2 siRNA (sense 5’-GAA GTG AAACTCA GGT CCG-3’; antisense 5’-AGT GAA ACAGTGC AGC TGC-3’) versus mouse TIPE2 mRNA and scrambled siRNA were purchased and synthesized (Shanghai Gene Pharma Co., Ltd). Six to eight weeks old C57BL/6 mice (n = 6) were injected with 5 OD/20gbw of TIPE2 siRNA or scrambled siRNA on day-3 through the tail vein. Transfection reagent kit of EntransterTM-in vivo (Engreen Biosystem, China) was employed to deliver the siRNA according to the manufacturer’s guidelines. To identify knockdown of Tipe2, plasm TIPE2 mRNA and protein were measured. Tipe2 siRNA mice showed a significant decrease of TIPE2 mRNA and protein content (Supplementary Figure S1, available in www.besjournal.com). Animals were accepted apocynin (4.8 mg/kg, Shanghai yousi Ltd., China) intraperitoneally on days -5, -4, -3, -2 and -1, followed by II/R. Lung tissue and blood samples were collected for further investigation.
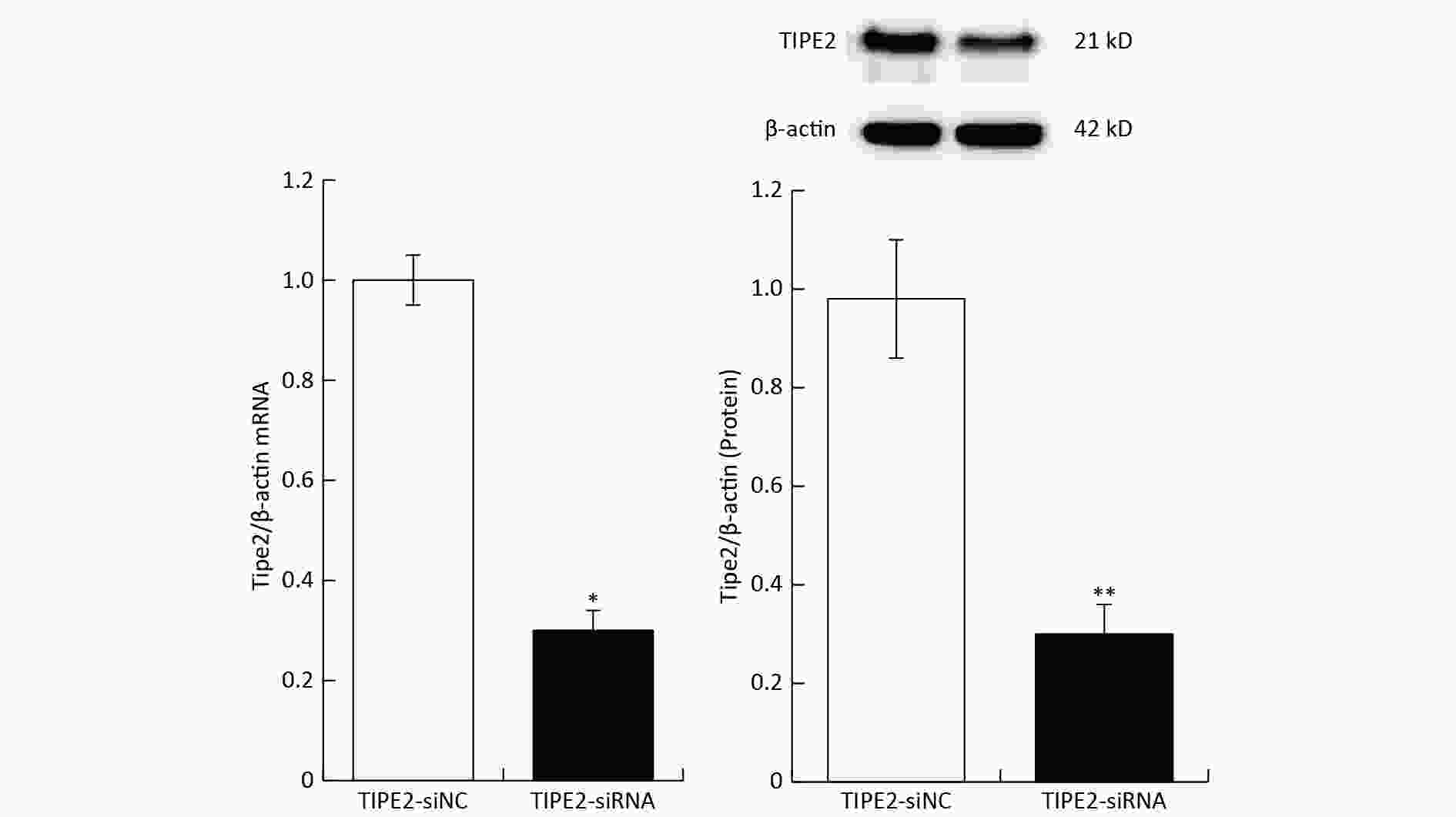
Figure S1. TIPE2 of plasm and intestinal TIPE2 in different groups (mean ± SD, n = 6). *P < 0.01 vs. TIPE2-siNC group by Student’s t test.
For detecting TIPE2, NF-κB p65, Nox-2 and phosphorylated-p66shc (p-p66shc) in lung tissue, 30 μg of cytoplasm, nucleus or total protein was extracted using 12%–15% concentrated sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Bio-Rad, Hercules, United States). Then Western Bloting was performed. Total RNA was acquired from animals with Trizol kits (Invitrogen, Carlsbad, CA) according to the manufacturer’s recommendations. RNA was transcribed into complementary DNA using an RNA polymerase chain reaction (PCR) Kit (TaKaRa, China); primers are appeared as follows: TIPE2 sense: 5’-GGGAACATCCAAGGCA AG-3’ (forward), reverse 5’-AGCTCATCTAGCACCTCACT-3’ (reverse). β-actin sense: forward 5’-ATTGGCAATGAGCGGTTCCG-3’ (forward), reverse 5’-AGG GCAGTGATCTCCTTCTG-3’ (reverse). Then Real-time PCR (RT-PCR) and fluorescence determination during amplification were performed.
All data were analyzed using SPSS22.0 software (SPSS, Chicago, IL). Data are expressed as mean ± SD. Two groups were performed using t-test. Statistical comparisons were analyzed by Kruskal–Wallis test for non-normal distributions followed by Wilcoxon Rank Sum test with Bonferroni adjustments for multiple comparisons, or a one-way analysis of variance (ANOVA) followed by Student– Newman–Keuls (SNK) test for normal distributions. Differences were considered significant when P < 0.05.
Apocynin has performed a strong effect against inflammatory and oxidant related disease[9]. Our present data provides novel example which parallel with previous agents[5,8]. Apocynin alleviates the lung morphologic hemorrhagic, neutrophil infiltration, and edema compared with the sham and model group, as well as the histopathological scores of lungs. In addition, Apocynin improves intestinal mucosal lesion with obvious bleeding and reduce of glands and villi of lamina propria compared with the sham and model group, also histopathological scores of intestine (Figure 1A–C). We further evaluate the lung W/D ratio and BALFP level. Apocynin significantly reduced the lung W/D ratio and BALFP levels in II/R animals compared with the sham and model group (Figure 1D–E). This indicates apocynin mitigates II/R-induced ALI remarkably.
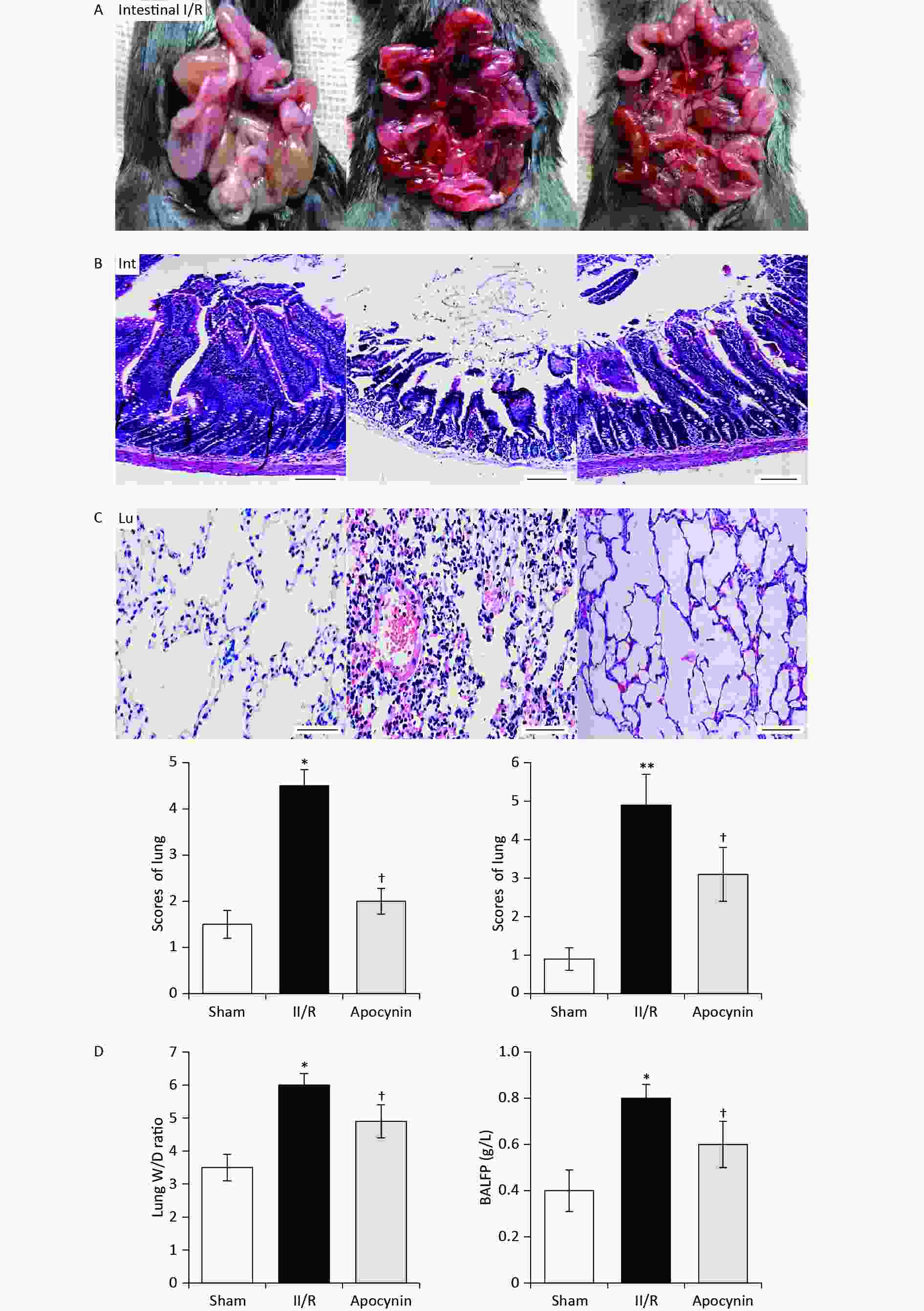
Figure 1. C57BL/6J mice intestinal intestinal ischemia/reperfusion (II/R) model (A) in vivo of sham group, II/R group, and apocynin group. Pathologic alterations of lung (B) and intestinal (C) in different groups (mean ± SD, n = 8) with hematoxylin-eosin (H&E) staining; scale bar: 100 μm. **P < 0.01 *P < 0.05 vs. sham group; †P < 0.05 vs. II/R group. Lung lung wet/dry (W/D) ratio (D) and BALFP levels (E) in different groups (mean ± SD, n = 8). *P < 0.05 vs. sham group; †P < 0.05 vs. II/R group. BALFP, bronchoalveolar lavage fluid protein.
NF-κB is the critical transcription factor which regulates the inflammatory cascade and activated during oxidative and various stress situations. NF-κB p65 translocated to the cellular nuclear is the represented event of NF-κB pathway activation[2]. Apocynin significantly reduced NF-κB p65 expression venus II/R and sham animals (Figure 2A). P66shc is a classical proapoptotic marker which mediated apoptosis via phosphorylation[2]. Apocynin decreased p-p66shc/p66shc ratio markedly versus II/R and sham animals Figure 2B). Furthermore, M1 cytokines MCP-1, TNF-α, IL-6, and IL-1β are involved in II/R-induced ALI and demonstrated previously. Apocynin lowered pro-inflammatory cytokines of MCP-1, TNF-α, IL-6, and IL-1β venus sham and II/R animals. (Figure 2C–F).
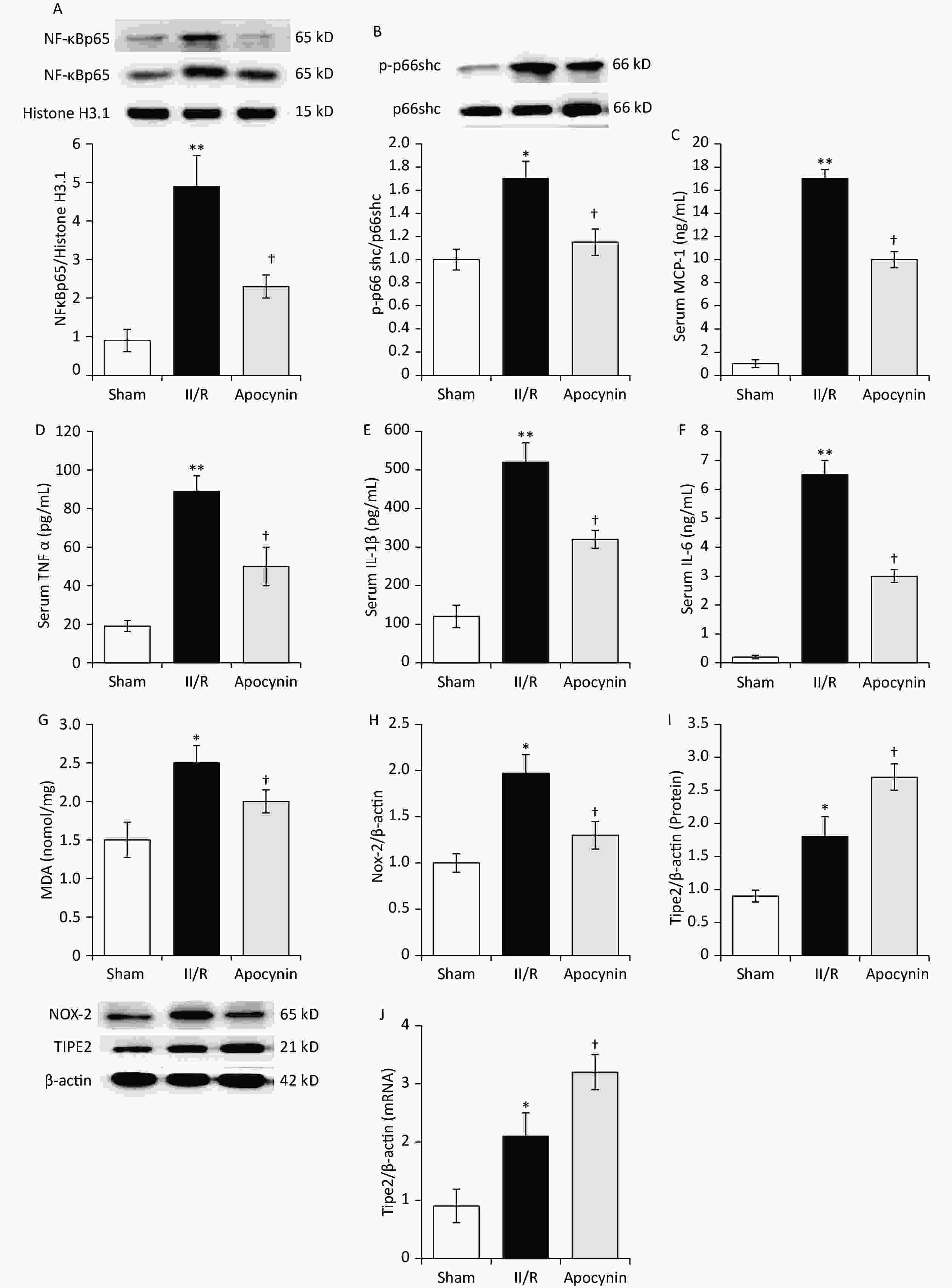
Figure 2. Western blot analysis of lung (A) NF-κ Bp65, (B) p-p66schc/p66shc ratio, ELISA analysis (C, D, E, F) serum MCP-1, TNF-α, IL-6 and IL-1β levels in different groups (mean ± SD, n = 8). *P < 0.05, **P < 0.01 vs. sham group; †P < 0.05 vs. intestinal ischemia/reperfusion (II/R) group. Lung tissue MDA content (G), Nox-2 (H) and TIPE2 protein (I) and mRNA (J) levels in different groups (mean ± SD, n = 8). *P < 0.05 vs. sham group; †P < 0.05 vs. II/R group. MDA, Malondialdehyde; ELISA, enzyme-linked immunosorbent assay.
Oxidative stress is another factor always associated with inflammatory cascade in this pathogenesis[1]. Various cells including endothelial cell, macrophages and neutrophils increase ROS, MDA, and Nox-2 during II/R[1,2]. In present study, apocynin decrease Nox-2 and MDA markedly (Figure 2G–H).
Moreover, TIPE2 protein and mRNA content were determined. Compared with sham group, lung TIPE2 protein and mRNA increased remarkably in II/R animals. Apocynin further increased TIPE2 protein and mRNA levels vesus II/R animals (Figure 2I–J). TIPE2 was discovered in accelerating M2 macrophage polarization while dampening M1 macrophage polarization[10]. Lack of TIPE2 caused leukocytosis and multiple organ inflammation in mice. Reduction of TIPE2 in human is suffered of systemic autoimmunity. These indicate TIPE2 is a pivotal target in controlling immune internal environment balance. Our study are consistent with previous results and provides convinced evidence of TIPE2 on lung protection against II/R.
In order to identity the essential role of TIPE2 on II/R-induced ALI, a variable of indicators, including histopathological scores, lung W/D ratio and BALFP, M1 subtype cytokine, as well as MDA and Nox-2, were measured. In addition, NF-κB p65 and p-p66shc/p66shc are evaluated. TIPE2-siRNA mice who suffered II/R showed a sever lung morphologic and deteriorate lung W/D ratio venus TIPE2-siNC II/R mice both apocynin treated. Moreover, M1 subtype cytokine (TNF-α and MCP-1), MDA and Nox-2, as well as NF-κB p65 and p-p66shc/p66shc are increased remarkably venus TIPE2-siNC II/R mice both apocynin treated (Figure 3A–E). This indicates that TIPE2 inhibits various deteriorate factors mentioned above in II/R-induced ALI. We seems for the first time draw the required and pivotal role of TIPE2 in apocynin mediated protection of ALI against II/R (Figure 4).
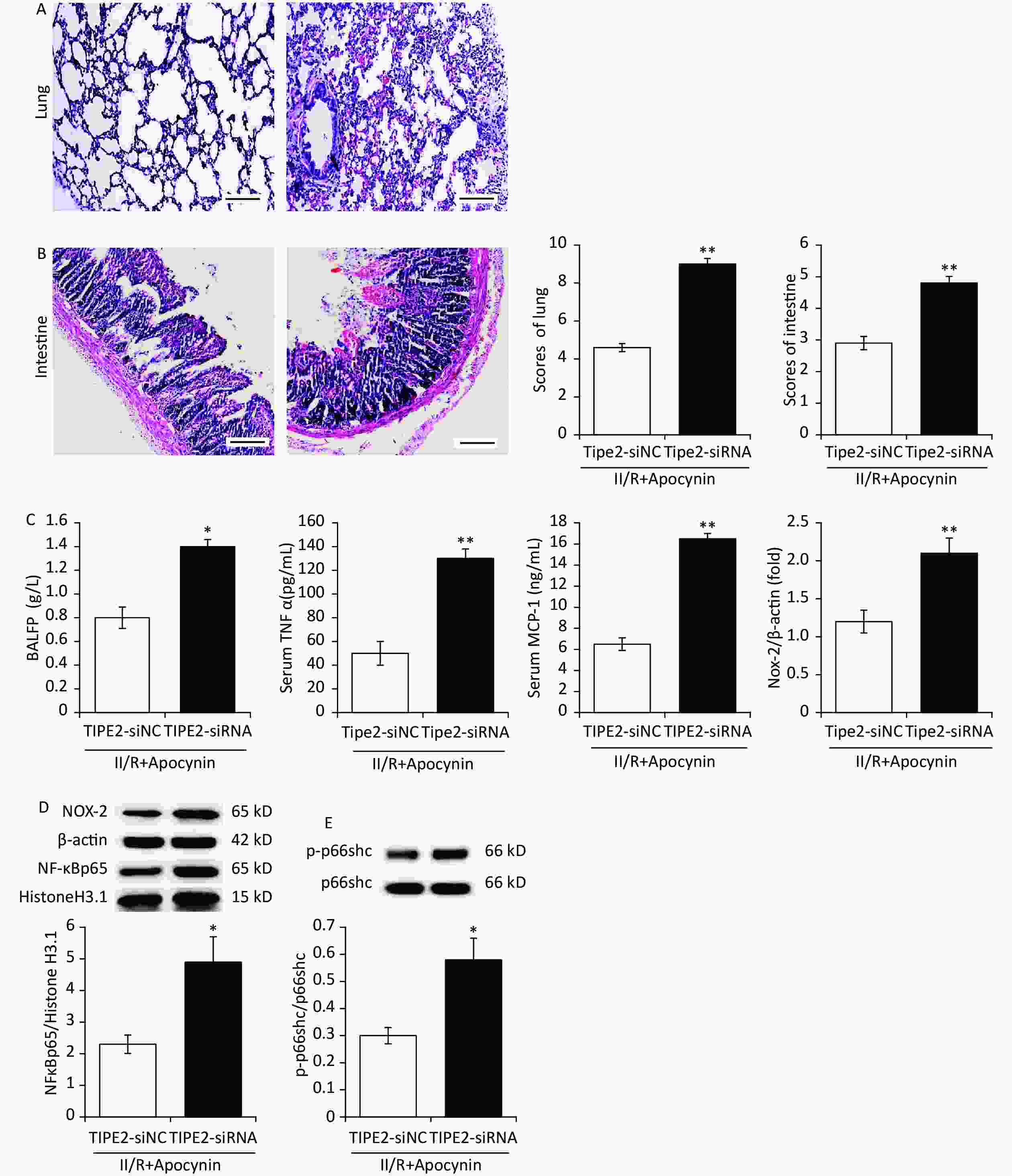
Figure 3. Apocynin ameliorates intestinal ischemia/reperfusion (II/R) induced acute lung and intestinal injurie in TIPE2-siRNA animals. Lung (A) and intestine (B) with hematoxylin-eosin (H&E) staining; (scale bar: 100 μm). Lung BALFP, Nox-2 and serum MCP-1, TNF-α (C) expression, TIPE2, NFκBp65 content (D) and p66shc/p66shc ratio (E) of lung in different groups (mean ± SD, n = 8). *P < 0.05 vs. sham group; †P < 0.05 vs. II/R group.
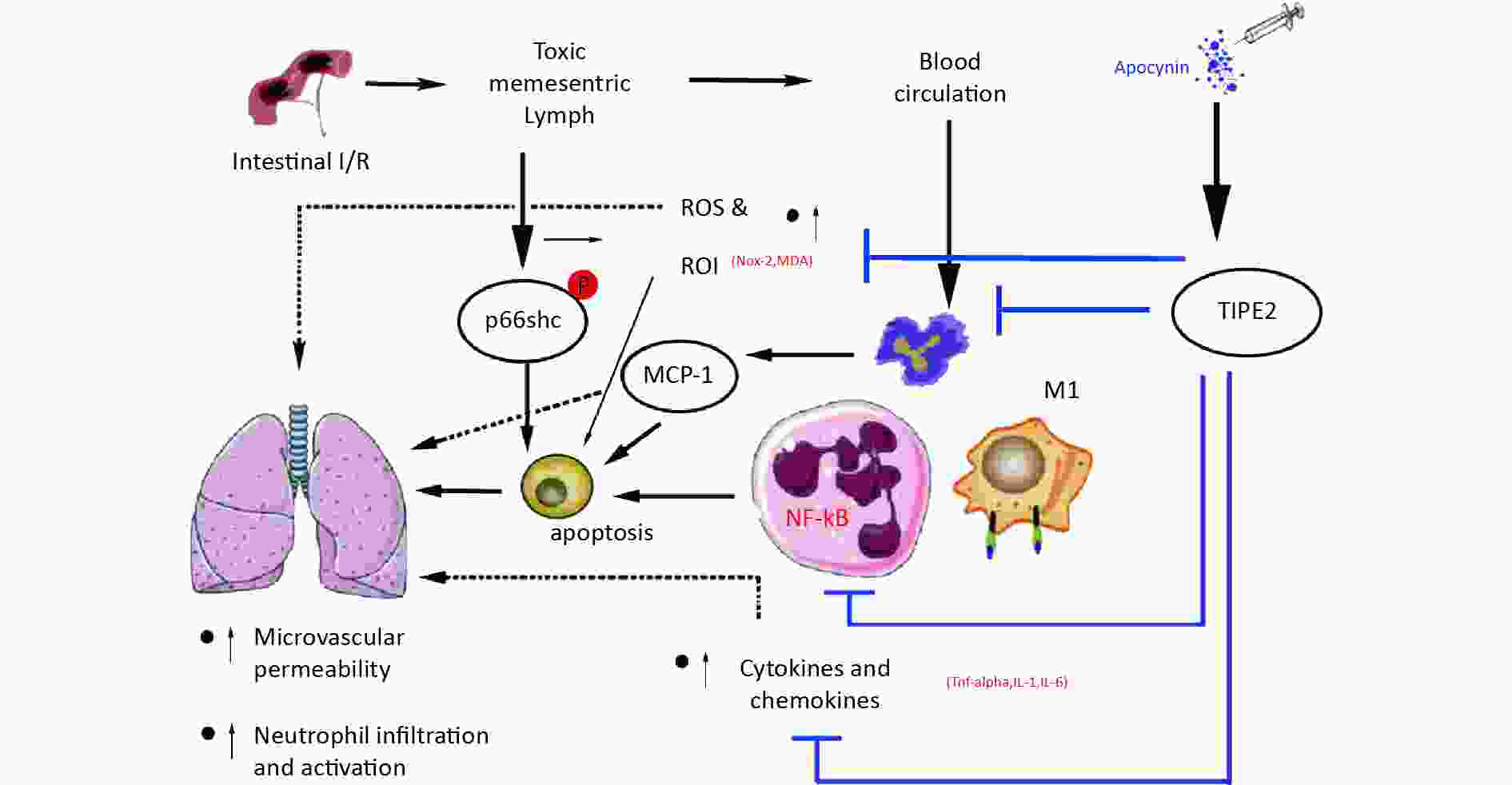
Figure 4. Schematic representation the novel potential protective mechanism of apocynin in lung injury induced by intestinal intestinal ischemia/reperfusion (II/R) through TIPE2 pathway.
In conclusion, our results suggest that apocynin protects against II/R-induced ALI by enhancing TIPE2 expression and suppressing NF-κB activation, M1 macrophage polarization, oxidative stress, and apoptosis. These findings are consistent with previous studies that showed the anti-inflammatory and anti-oxidative effects of apocynin in various models of tissue injury However, our study has some limitations, such as the lack of a direct measurement of TIPE2 activity, the use of a single dose and route of apocynin administration, and the short-term observation of the outcomes. Further studies are needed to elucidate the molecular mechanisms of TIPE2 and apocynin in II/R-induced ALI and to optimize the therapeutic regimen and efficacy of apocynin in clinical settings.
doi: 10.3967/bes2024.124
TIPE2 is Essential for Apocynin-mediated Protection against Intestinal Ischemia/Reperfusion-induced Acute Lung Injury
-
The authors declare that they have no conflicts of interest.
注释:1) Conflicts of Interest: -
Figure 1. C57BL/6J mice intestinal intestinal ischemia/reperfusion (II/R) model (A) in vivo of sham group, II/R group, and apocynin group. Pathologic alterations of lung (B) and intestinal (C) in different groups (mean ± SD, n = 8) with hematoxylin-eosin (H&E) staining; scale bar: 100 μm. **P < 0.01 *P < 0.05 vs. sham group; †P < 0.05 vs. II/R group. Lung lung wet/dry (W/D) ratio (D) and BALFP levels (E) in different groups (mean ± SD, n = 8). *P < 0.05 vs. sham group; †P < 0.05 vs. II/R group. BALFP, bronchoalveolar lavage fluid protein.
Figure 2. Western blot analysis of lung (A) NF-κ Bp65, (B) p-p66schc/p66shc ratio, ELISA analysis (C, D, E, F) serum MCP-1, TNF-α, IL-6 and IL-1β levels in different groups (mean ± SD, n = 8). *P < 0.05, **P < 0.01 vs. sham group; †P < 0.05 vs. intestinal ischemia/reperfusion (II/R) group. Lung tissue MDA content (G), Nox-2 (H) and TIPE2 protein (I) and mRNA (J) levels in different groups (mean ± SD, n = 8). *P < 0.05 vs. sham group; †P < 0.05 vs. II/R group. MDA, Malondialdehyde; ELISA, enzyme-linked immunosorbent assay.
Figure 3. Apocynin ameliorates intestinal ischemia/reperfusion (II/R) induced acute lung and intestinal injurie in TIPE2-siRNA animals. Lung (A) and intestine (B) with hematoxylin-eosin (H&E) staining; (scale bar: 100 μm). Lung BALFP, Nox-2 and serum MCP-1, TNF-α (C) expression, TIPE2, NFκBp65 content (D) and p66shc/p66shc ratio (E) of lung in different groups (mean ± SD, n = 8). *P < 0.05 vs. sham group; †P < 0.05 vs. II/R group.
-
[1] Pastores SM, Katz DP, Kvetan V. Splanchnic ischemia and gut mucosal injury in sepsis and the multiple organ dysfunction syndrome. Am J Gastroenterol, 1996; 91, 1697−710. [2] Wang GZ, Yao JH, Jing HR, et al. Suppression of the p66shc adapter protein by protocatechuic acid prevents the development of lung injury induced by intestinal ischemia reperfusion in mice. J Trauma Acute Care Surg, 2012; 73, 1130−7 doi: 10.1097/TA.0b013e318265d069 [3] Silva JTC, Fonseca Neto OCLD. Acute mesenteric ischemia and COVID-19: an integrative review of the literature. Rev Col Bras Cir, 2023; 50, e20233334. [4] Xu MM, Kang JY, Ji S, et al. Melatonin suppresses macrophage M1 polarization and ROS-mediated pyroptosis via activating ApoE/LDLR pathway in influenza A-induced acute lung injury. Oxid Med Cell Longev, 2022; 2022, 2520348. [5] Zhang Y, Wei XB, Liu LX, et al. TIPE2, a novel regulator of immunity, protects against experimental stroke. J Biol Chem, 2012; 287, 32546−55. doi: 10.1074/jbc.M112.348755 [6] Du YM, Liu XH, Xiao CJ, et al. TIPE2 regulates periodontal inflammation by inhibiting NF-κB p65 phosphorylation. J Appl Oral Sci, 2023; 31, e20230162. doi: 10.1590/1678-7757-2023-0162 [7] Kong Q, Yuan M, Ming TQ, et al. Expression and regulation of tumor necrosis factor-α-induced protein-8-like 2 is associated with acute lung injury induced by myocardial ischemia reperfusion in diabetic rats. Microvasc Res, 2020; 130, 104009. doi: 10.1016/j.mvr.2020.104009 [8] Pintard C, Ben Khemis M, Liu D, et al. Apocynin prevents GM-CSF-induced-ERK1/2 activation and -neutrophil survival independently of its inhibitory effect on the phagocyte NADPH oxidase NOX2. Biochem Pharmacol, 2020; 177, 113950. doi: 10.1016/j.bcp.2020.113950 [9] Chan SMH, Brassington K, Almerdasi SA, et al. Inhibition of oxidative stress by apocynin attenuated chronic obstructive pulmonary disease progression and vascular injury by cigarette smoke exposure. Br J Pharmacol, 2023; 180, 2018−34. doi: 10.1111/bph.16068 [10] Zhao SJ, Shen YK, Wu SN, et al. TIPE2 inhibits MGD inflammation by regulating macrophage polarization. J Pers Med, 2023; 13, 492. doi: 10.3390/jpm13030492 -
 24016+Supplementary Materials.pdf
24016+Supplementary Materials.pdf

-




 下载:
下载:





 Quick Links
Quick Links