-
Acute lung injury (ALI) is a common complication in patients with extensive deep burns[1,2]. Once ALI occurs, patients become susceptible to acute respiratory distress syndrome (ARDS), which is associated with high mortality[3-5]. Currently, there are no effective therapeutic strategies for treating ALI following severe burns. Hence, elucidating the molecular mechanisms underlying ALI progression is vital for discovering potential therapeutic measures. ALI is characterized by excessive and uncontrolled inflammation mediated by multiple immune cells[5-7]. Inflammatory cell infiltration and the release of inflammatory mediators are the main causes of ALI, and reducing inflammation is effective in improving ALI outcomes[8,9].
Accumulating evidence indicates that mesenchymal stem cells (MSCs) ameliorate ALI by inhibiting inflammatory responses, suggesting a promising strategy for preventing and treating ALI[10-12]. Further investigations have revealed that exosomes derived from MSCs provide therapeutic benefits[13,14]. Exosomes are extracellular vesicles secreted from cells via exocytosis, capable of modulating biological signals by transferring loaded cargo (e.g., non-coding RNA, mRNA, lipids, and proteins) to recipient cells[15]. Recent studies have revealed that microRNA (miRNA) from MSCs exosomes attenuates ALI[16,17]. The miRNAs are a type of non-coding RNA that can post-transcriptionally suppress their levels by targeting mRNA. Various miRNAs play roles in mitigating inflammation in ALI[18-20]. In a previous study, we demonstrated that miR-451 from human umbilical cord MSCs (hUC-MSCs) weakened burn-induced inflammatory responses and alleviated ALI[21]. However, its underlying mechanisms are not yet fully understood.
When ALI occurs, numerous immune cells, including neutrophils and macrophages, migrate into the lung tissues. As the main functional cells of the pulmonary immune system, alveolar macrophages are the first barrier against ALI and play a critical role in its onset and progression[22]. Deng et al. discovered a notable reduction in miR-451 expression in the lungs of mice with ALI. Following treatment with A. manihot capsules, miR-451 expression was upregulated[23]. Our previous work clarified that exosomal miR-451 in hUC-MSCs improved ALI by promoting macrophage M2 polarization[24]. However, the effects of alveolar macrophages on miR-451-mediated ALI alleviation and the underlying mechanisms remain unclear. Increasing studies have indicated that autophagy is a key pathogenic mechanism in ALI and occurs in lung injury caused by various factors[25,26]. Autophagy inhibitor, 3-methyladenine, may inhibit autophagy in alveolar macrophages, thereby alleviating ALI[27]. Hydrogen-rich saline ameliorates ALI by inhibiting autophagy[28]. Thus, modulation of alveolar macrophage autophagy may be a potential strategy for ALI therapy. However, whether the effect of miR-451 on ALI is dependent on autophagy in alveolar macrophages remains unclear. It has been documented that miR-451 can regulate cell autophagy by targeting tuberous sclerosis complex 1 (TSC1)[29]. There is substantial evidence that mammalian target of rapamycin (mTOR) signaling plays a vital role in autophagy inhibition. TSC1 suppresses the mTOR pathway, thereby promoting autophagy[30]. Therefore, we hypothesized that miR-451 from hUC-MSC exosomes may improve ALI by restricting alveolar macrophage autophagy through the TSC1/mTOR signaling pathway.
This study aimed to investigate the underlying mechanism of hUC-MSC-derived exosomal miR-451 in attenuating ALI. First, in vivo study to determine the effect of miR-451 from human umbilical cord mesenchymal stem cell-derived exosomes (hUC-MSC-Exos) on autophagy. An in vitro study confirmed that hUC-MSC exosomes regulate autophagy by delivering miR-451 to alveolar macrophages. Finally, the molecular mechanisms underlying miR-451-regulated autophagy in ALI were elucidated. Thus, this study provides a novel therapeutic strategy for the treatment of ALI.
-
Human umbilical cord tissue was obtained from healthy donors who delivered at the First Affiliated Hospital of Anhui Medical University and provided informed consent. All experimental protocols were approved by the ethics committee. The hUC-MSCs were isolated and cultured as previously described in our work[24]. The hUC-MSCs used for exosome extraction were cultured in Dulbecco's modified Eagle’s medium (DMEM; Hyclone, Utah, USA) with 10% exosome-depleted fetal bovine serum (FBS; SBI System Biosciences, Shanghai, China). Human embryonic kidney293T (HEK293T) cells were routinely cultured in DMEM supplemented with 10% FBS (Gibco, Grand Island, New York, USA).
Alveolar macrophages were isolated from burn rat models and normal rats. Sprague-Dawley (SD) rats (male, 6–8 weeks old) were purchased from the Comparative Medicine Center of Yangzhou University. After feeding for one week under specific pathogen-free conditions, macrophages were extracted from the alveolar lavage fluid directly or after constructing the burn model. For the former, the macrophages were treated with 10 ng/mL lipopolysaccharide (LPS) (Sigma-Aldrich, Missouri, USA) and/or the corresponding exosomes, followed by further experiments. Macrophages were only used for western blot analysis.
-
Cell transfection was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, California, USA) according to the manufacturer’s instructions. Passage 2 alveolar macrophages from normal rats were transfected with a miR-451 mimic and inhibitor, si-TSC1, and the respective controls obtained from HEMA Probe Technology (Huzhou, China). Passage 3 hUC MSCs were transfected with miR-451 and a control inhibitor, followed by exosome isolation. For luciferase assay, the TSC1-3' untranslated regions (UTR) was cloned into pmirGLO vector (KeyGen Technology, Nanjing, China) to form wildtype (WT). reporter plasmid Meanwhile, mutant TSC1-3' UTR reporter plasmid (MUT) was constructed based on TSC1-3'UTR-WT. Reporter plasmids were provided by KeyGen Technology (Nanjing, China). HEK293T cells were co-transfected with miR-451 mimics and reporter plasmids. To inhibit mTOR, alveolar macrophages were pretreated with 100 nmol/L rapamycin (HY-10219, MedChemExpress, New Jersey, USA) for 1 h prior to transfection. si-TSC1, miR-451 mimic and inhibitor sequences are listed in Supplementary Table S1 (available in www.besjournal.com).
Table S1. Sequences used in cell transfection
shRNA name Sequences si-TSC1-#1 Sense: GGUGUUGAUCACCAUGCUACC
Antisense: UAGCAUGGUGAUCAACACCAAsi-TSC1-#2 Sense: CGGCUGAUGUUGUUCAGUACC
Antisense: UACUGAACAACAUCAGCCGAGsi-TSC1-#3 Sense: GACAGUAUAGAGAAAGAUAAG
Antisense: UAUCUUUCUCUAUACUGUCUUmiR-451 mimic Sense: AAACCGUUACCAUUACUGAGUU
Antisense: AACUCAGUAAUGGUAACGGUUUmiR-451 inhibitor AACUCAGUAAUGGUAACGGUUU -
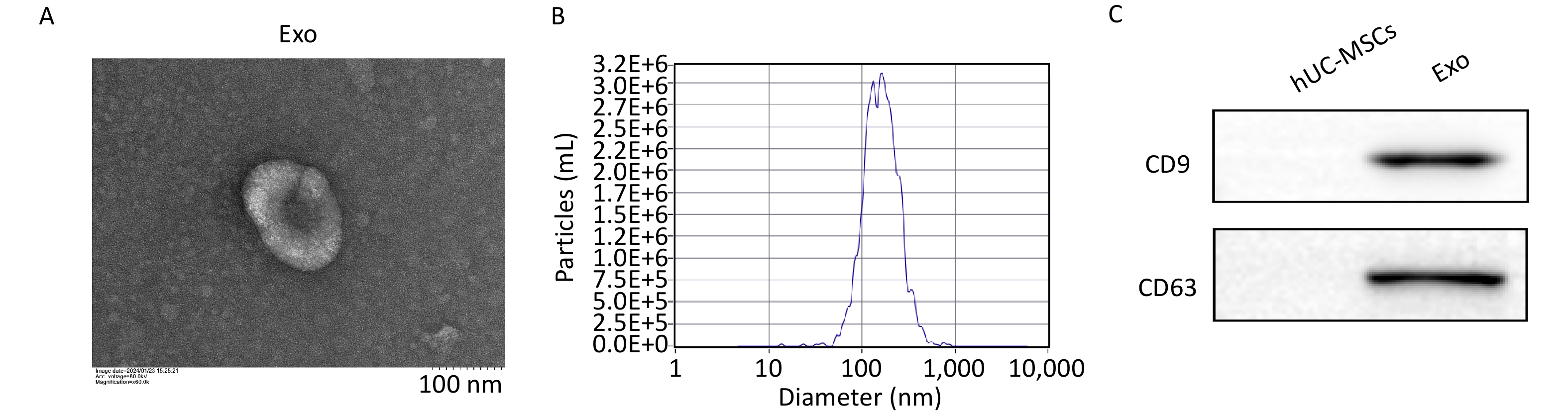
Exosomes were extracted from the hUC-MSC supernatant using the Total Exosome Isolation Reagent (Invitrogen, California, USA), according to the manufacturer’s instructions. Briefly, the supernatant was centrifuged at 2,000 ×g for 30 min to remove cells and debris. The supernatant was then mixed with the isolation reagent and incubated at 4 °C overnight. After the mixture was centrifuged at 10,000 ×g for 60 min, the precipitate was dissolved in 200 μL phosphate-buffered saline (PBS) to obtain the exosomes. The structural and morphological features of exosomes were examined using a transmission electron microscope (Hitachi HT-7700, Japan). The particle size distribution and concentration of exosomes were determined by nanoparticle tracking analysis. Western blotting was performed to detect the presence of exosomal markers CD9 (20597-1-AP, Proteintech) and CD63 (25682-1-AP, Proteintech). The exosome morphology, size distribution, and surface marker expression results are depicted in Supplementary Figure S1 (available in www.besjournal.com) .
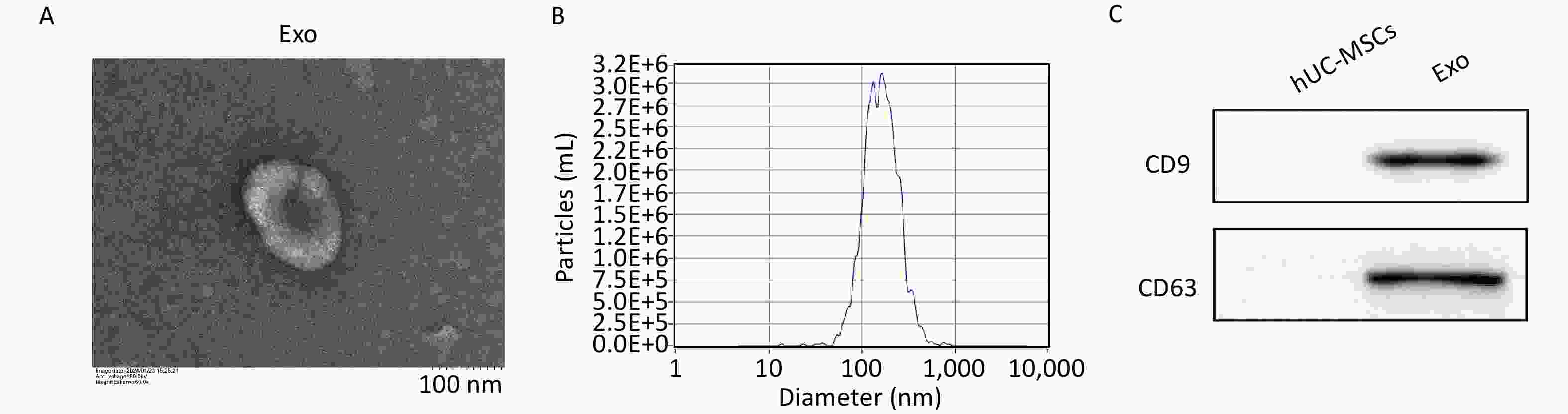
Figure S1. Characterization of hUCMSC-Exos. A, The ultrastructure of hUCMSC-Exo was analyzed by transmission electron microscopy. Scale bar = 100 nm. B, Measurement of hUCMSC-Exos size distribution by Nanoparticle Tracking Analysis. C, Expression of the protein markers CD9 and CD63, which are commonly found in exosomes, was determined by western blot.
-
Severe burn-induced ALI models was stimulated using third-degree burns covering 30% of the total body surface area (TBSA) with full-thickness burn wounds, as previously described[21]. Rats were randomly assigned to six groups (n = 3/group): Control; ALI; ALI + PBS (injection of PBS); ALI + Exo (injection of exosomes); ALI + NCI-Exo (injection of exosomes transfected with a control inhibitor); and ALI + miR-451I-Exo (injection of exosomes transfected with an miR-451 inhibitor). Exosomes were administered via intravenous injection into the rat's tail vein, at a volume of 100 μL. After 48 h of treatment, the animals were euthanized using carbon dioxide. Lung tissues and bronchoalveolar lavage fluid (BALF) were collected for further experiments. Collection of BALF involved making a longitudinal incision in the neck skin and peritoneum covering the thyroid to expose the trachea. This was followed by a small diagonal cut using scissors, as distally as possible from the trachea. A catheter was inserted through the mouth and securely tied using a cotton thread. Through this, 1 mL of pre-cooled PBS was slowly instilled and withdrawn three times to collect BALF, resulting in a recovery rate of 70%–80%. For rapamycin treatment, rats were randomly divided into four groups (n = 3/group): Control, ALI, ALI+si-TSC1, and ALI+si-TSC1+ rapamycin. In the ALI+si-TSC1 group, rats were administered TSC1-inhibiting lentivirus via tail vein injection after ALI modeling. In the ALI+si-TSC1+ rapamycin group, rats were administered 1 mg/(kg∙day) of rapamycin for three consecutive days before ALI modeling, followed by tail vein injection of TSC1-inhibiting lentivirus after modeling. All the procedures were conducted following approval by the First Affiliated Hospital of Anhui Medical University ethics committee.
-
Total RNA was isolated from hUC-MSC exosomes (hUC-MSC-Exo) and alveolar macrophages using TRIzol reagent (Biosharp, Hefei, China), followed by reverse transcription into cDNA. For miR-451, cDNA was synthesized using the HiScript II Reverse Transcription Kit (Vazyme, Nanjing, China) with the stem-loop primer, specific to miR-451. For TSC1, reverse transcription was performed using the Hifair II 1st Strand cDNA Synthesis Kit (Cronda, Shanghai, China) with random primer N6. The qRT-PCR was conducted to amplify miR-451 and TSC1 using 2 × ChamQ Universal SYBR qPCR Master Mix (Vazyme) and Hieff ®qPCR SYBR Green Master Mix (Cronda), respectively. U6 and β-actin were used as internal controls for miR-451 and TSC1, respectively. Each assay was conducted in triplicate and repeated thrice. Relative expression levels were calculated using the 2−ΔΔCT method. The primers for miR-451 were designed using miRNA Design (Vazyme), and the primers for TSC1 were designed using Primer Premier 5.0 (Sangon, Shanghai, China), respectively. All primers were synthesized by Sangon (Shanghai, China), and the sequences are listed in Supplementary Table S2, available in www.besjournal.com.
Table S2. Primer sequences for qRT-PCR
Gene Sequences miR-451-RT 5’- CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAACTCAGT-3’ miR-451 F: 5’-GCCGGAAACCGTTACCATT-3’ URP 5’-CTCAACTGGTGTCGTGGA-3’ U6-RT F: 5’-CTCGCTTCGGCAGCACA-3’
R: 5’-AACGCTTCACGAATTTGCGT-3’TSC1 F: 5’-GTAAACACGTTGGTGGATT-3’
R: 5’-GCTTTGCCTACATACTCATTC-3’β-actin F: 5’-GTCCCTCACCCTCCCAAAAG-3’
R: 5’-GCTGCCTCAACACCTCAACCC-3’ -
Total protein was extracted from the macrophages using RIPA buffer (Biosharp) and analyzed using western blotting. Protein samples were separated using 12% sodium dodecyl-sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Millipore, USA). After blocking with 5% skimmed milk in tris-buffered saline, 0.1% Tween 20 for 2 h, the membranes were incubated overnight at 4 °C with primary antibodies specific to Beclin-1 (Proteintech, Rosemont, IL, USA, # 11306-1-AP); Lc3 II/I (Proteintech, #14600-1-AP); TSC1 (Abcam, ab27096); mTOR (Proteintech, #28273-1-AP); P-mTOR (Proteintech, #67778-1-Ig); ATG13 (Abcam, Cambridge, UK, #ab201467); ULK1 (Proteintech, #29005-1-AP); p-ULK1 (Proteintech, #30005-1-AP); and β-actin (Abcam, #ab270967). Following incubation in secondary antibodies at room temperature for 1 h, the blots were scanned using the imaging system (BioRad, California, USA) and analyzed using Image J software. β-actin was used as the loading control.
-
ELISA assays were performed to quantify the levels of LPS (COIBO, Shanghai, China, #CB11038-Ra); TNF-α (Elabscience, Wuhan, China, #E-EL-R2856c); and IL-6 (Elabscience, #E-EL-R0015c) in lung tissues and alveolar lavage fluid from burn rat models. Standard procedures were followed using three biological replicates for each treatment group. The optical density was measured at 450 nm using a microplate reader (I3, Molecular Devices, Silicon Valley, USA) and the concentrations of LPS, TNF-α, and IL-6 were calculated.
-
HE staining was performed as previously described[24]. Lung tissues were fixed in 10% formalin, dehydrated, embedded in paraffin, and sectioned. After deparaffinization and rehydration, the sections were stained with hematoxylin for 3–5 min. Following differentiating for 5–10 s, the sections were returned to blue for 20 s and then counterstained with eosin for 1 min. Running water was used to rinse the slices after the operation during the above steps. Finally, the sections were dehydrated, sealed, and imaged under a microscope (Leica, DMI3000B).
-
Macrophages were seeded onto 48-well plates containing coverslips. After fixation with 4% paraformaldehyde and permeabilization with 1% Triton X-100, cells were blocked by 5% bovine serum albumin. Subsequently, they were incubated overnight 4 °C with rabbit anti-LC3 (Proteintech, #18725-1-AP). Following incubation with FITC-IgG (Servicebio, #GB22403), the nuclei were stained with Hoechst 33258 (Beyotime, Shanghai, China, #C1017). Finally, fluorescent images were captured using a confocal microscope (Leica, Wetzlar, Germany, DMI8).
-
Exosomal proteins from the hUC-MSCs were quantified using a bicinchoninic acid kit (Biosharp). A total of 100 μg of exosomes were incubated with 50 μL 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (Dil) dye (1μM, Umibio, #UR21017) for 10 min to label hUC-MSCs-Exo, followed by washing in 10 mL PBS. The labeled exosomes were extracted using ultra-high-speed centrifugation to remove excess dye. The precipitate was dissolved in 200 μL PBS, and the labeled exosomes without dye were obtained. Alveolar macrophages were cultured in a medium containing the labeled exosomes for 24 h. After removing the supernatant, the cells were washed with PBS and imaged under a confocal microscope (Leica).
-
The effect of miR-451 on the luciferase activity of TSC1 cells was assessed using a dual-luciferase reporter assay kit (Promega, USA). In 48-well plates, HEK293T cells were co-transfected with miR-451/control mimics and WT/MUT reporter plasmids for TSC1 together with a Renilla plasmid. After 48 h, cells were collected, and lysed in 50 μL Passive Lysis Buffer for 15 min. To determine the relative luciferase activity,
20 μL of the sample was mixed with 100 μL of firefly luciferase assay reagent, relative light unit 1 (RLU1) was measured. Subsequently, 100 μL Renilla luciferase assay reagent was added and RLU2 was measured. The ratio of RLU1 (firefly signal) to RLU2 (Renilla signal) was calculated.
-
Data are shown as mean ± standard deviation, and processed with GraphPad Prism 8.0. Comparisons between two groups were performed using Student’s t-test. One-way analysis of variance (ANOVA) was used to analyze differences among multiple groups. The criterion for a significant difference was set at P-value < 0.05.
-
To investigate the effect of hUC-MSC-exosome miR-451 on ALI, we first treated hUC-MSCs with an miR-451 inhibitor, followed by exosome extraction. Compared to hUC-MSC-Exos transfected with the control inhibitor (NCI-Exos), miR-451 was significantly downregulated in hUC-MSC-Exos treated with the miR-451 inhibitor (miR-451I-Exo, Figure 1A). After the burn rat models were successfully established to simulate ALI in vivo (Figure 1B), ELISA was performed to examine the levels of inflammatory factors in the lung tissues and alveolar lavage fluid. The concentrations of LPS, TNF-α, and IL-1β were significantly elevated after ALI, while their expressions were dramatically declined by exposure to hUC-MSCs-Exo (Figure 1C and D). Notably, when miR-451 expression was inhibited, the decrease caused by hUC-MSC-Exos was partially reversed (Figure 1C and D). Further histological evaluation demonstrated that the administration of hUC-MSC-Exos markedly inhibited inflammatory cell infiltration and injury resulting from ALI, which was partially abolished by treatment with the miR-451 inhibitor (Figure 1E). These data indicate that hUC-MSC exosomal miR-451 could alleviate burn-induced injury. Given that autophagy is a vital pathogenesis of ALI, the expression of autophagy-related proteins was evaluated after the isolating alveolar macrophages from burn rat models using different treatments. The results suggested a pronounced increase in Beclin-1 and LC3-II/LC3-I in the ALI group compared to the control group (Figure 1F), indicating higher autophagy levels after ALI. Furthermore, hUC-MSC exosomes significantly reduced ALI-induced autophagy, which was rescued by the miR-451 inhibitor. Thus, these findings reveal that miR-451 from hUC-MSC-Exos may improve ALI by suppressing autophagy in alveolar macrophages.
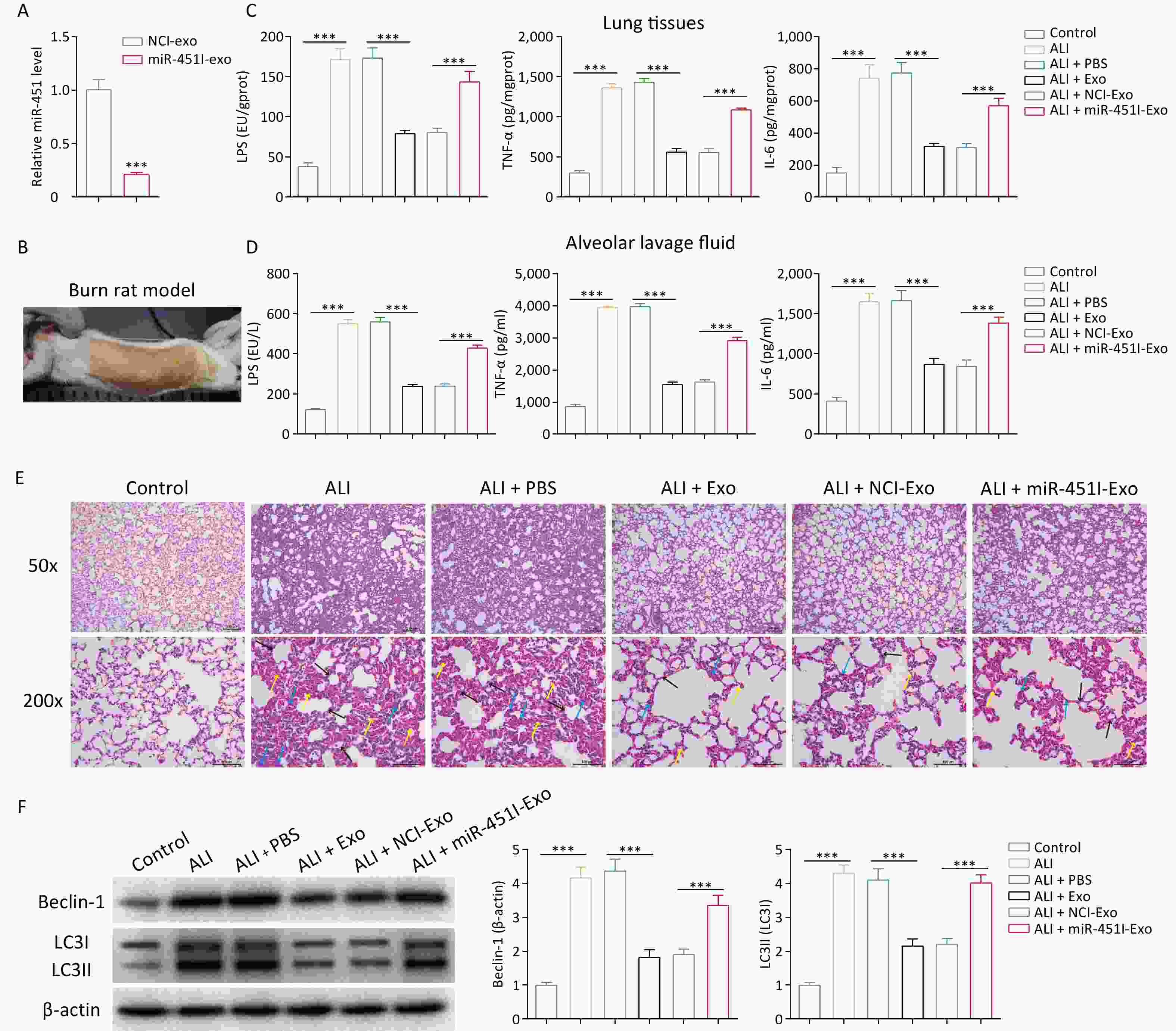
Figure 1. miR-451 from hUC-MSCs-Exo improves ALI through suppressing autophagy in alveolar macrophages. (A) Level of miR-451 in hUC-MSC-Exos was detected using qRT-PCR after treatment with the miR-451 inhibitor. (B) We established a rat burn model to simulate ALI in vivo. (C–D) ELISA was performed to examine the contents of LPS, TNF-α and IL-1β in lung tissues and alveolar lavage fluid of burn rats from different treatments. Hematoxylin and eosin staining was used to evaluate inflammatory cell infiltration and injury. Black arrows indicate interstitial lung swelling, yellow arrows indicate erythrocyte exudation, and blue arrows indicate inflammatory cell infiltration. (F) Protein expression of Beclin-1 and LC3-II/LC3-I in alveolar macrophages from rats with burns was tested using western blotting. All experiments were performed in triplicate (***P < 0.001). ALI, acute lung injury; LPS, lipopolysaccharide; hUC-MSC-Exo, human umbilical cord mesenchymal stem cell-derived exosomes; PBS, phosphate-buffered saline; NCI-Exo, exosomes transfected with a control inhibitor; miR-451I-Exo, exosomes transfected with an miR-451 inhibitor.
-
To explore whether hUC-MSC-derived exosomes regulated autophagy by delivering miR-451, alveolar macrophages from rats were treated with LPS to simulate ALI in vitro. A distinct reduction in miR-451 expression in alveolar macrophages was observed in the LPS group compared to the control group (Figure 2A). However, in alveolar macrophages, hUC-MSC-Exos promoted miR-451 expression, while miR-451 levels were substantially reduced when hUC-MSC-Exos were transfected with the miR-451 inhibitor (Figure 2A). These results suggest the possible transfer of miR-451 from hUC-MSCs to alveolar macrophages through exosomes. To verify this discovery, DiI was used to label hUC-MSC-Exos to determine the phagocytosis of exosomes by alveolar macrophages. As expected, exosomes from hUC-MSCs labeled with DiI were observed in alveolar macrophages (Figure 2B), indicating that macrophages phagocytose exosomes. Further investigations were performed to evaluate the role of hUC-MSC exosome miR-451 in macrophage autophagy in vitro using GFP-LC3 immunofluorescence staining. The results showed that autophagy was enhanced by LPS treatment (Figure 2C and D). Autophagy was inhibited by the addition of exosomes, and miR-451 inhibitor reversed this effect (Figure 2C and D). Similarly, the expression of Beclin-1 and LC3-II/LC3-I was greatly decreased in alveolar macrophages treated with exosomes, whereas this effect was rescued by treatment with the miR-451 inhibitor (Figure 2E). Collectively, these results illustrate that miR-451 suppresses autophagy by transferring hUC-MSCs to alveolar macrophages via exosomes.
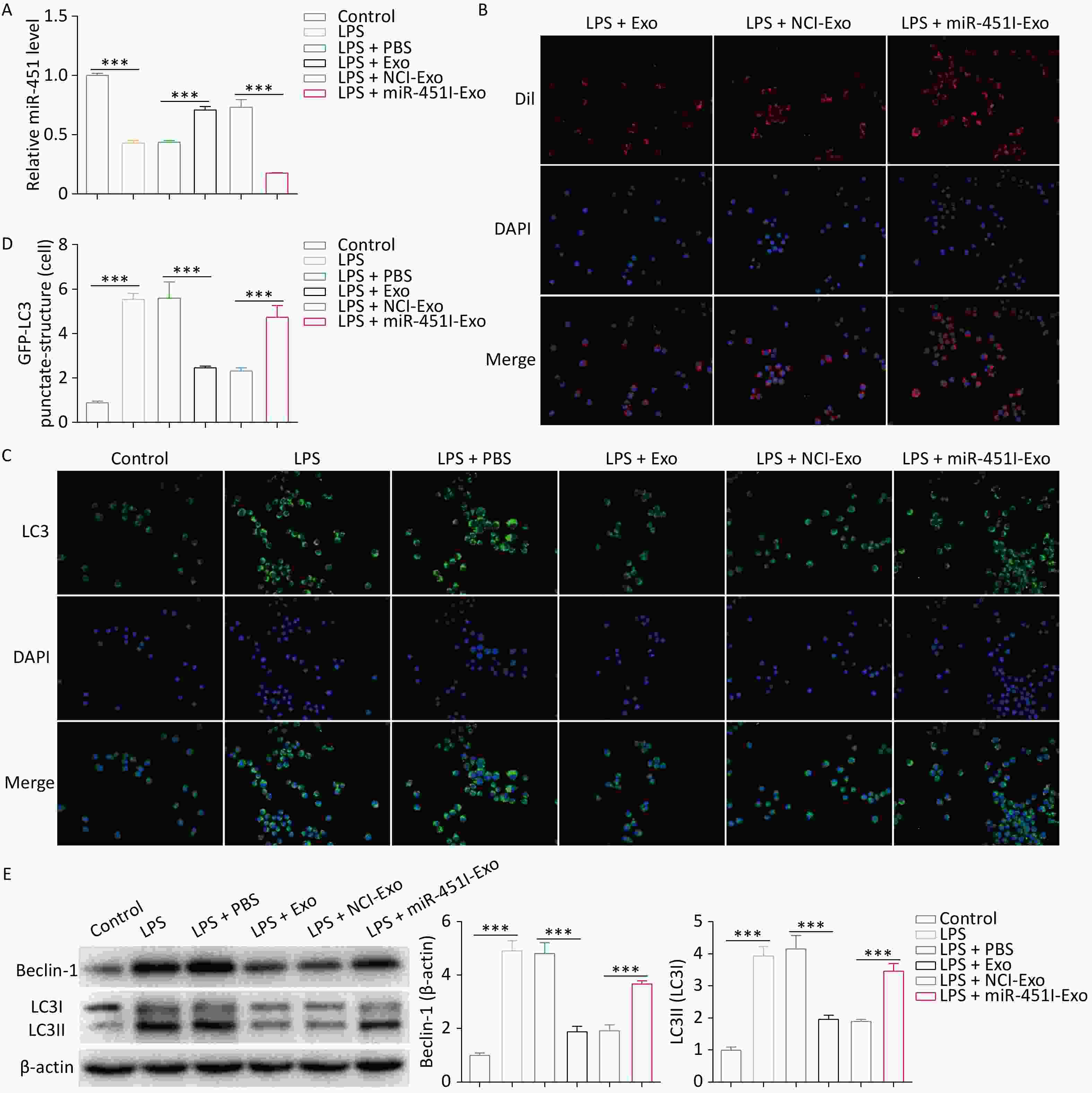
Figure 2. Alveolar macrophage autophagy is inhibited by miR-451 transferred by hUC-MSCs-Exo. (A) miR-451 expression in alveolar macrophages after LPS exposure and distinct hUC-MSC-Exo treatments was determined by qRT-PCR. (B) Immunofluorescence staining was performed to label hUC-MSC-Exos using Dil. (C–D) The effects of miR-451 from hUC-MSC-Exos on macrophage autophagy were evaluated using GFP-LC3 immunofluorescence staining. (E) Western blotting was performed to examine the protein levels of Beclin-1 and LC3-II/LC3-I in alveolar macrophages. All experiments were performed in triplicate (***P < 0.001). ALI, acute lung injury; LPS, lipopolysaccharide; hUC-MSC-Exo, human umbilical cord mesenchymal stem cell-derived exosomes; PBS, phosphate-buffered saline; NCI-Exo, exosomes transfected with a control inhibitor; miR-451I-Exo, exosomes transfected withan miR-451 inhibitor.
-
The above evidence clarifies the effect of miR-451 on autophagy; however, the underlying mechanism remains unclear. TSC1, an autophagy-related protein, is a miR-451[29]. The present work predicted the putative binding site of miR-451 in TSC1 3’UTR region using TargetScan[31] (Figure 3A). To determine whether miR-451 targets TSC1, wild-type and mutant reporter plasmids for TSC1 were generated. Dual-luciferase reporter analysis indicated that, compared to the mimic negative control (mimic NC) group, lower luciferase activity of TSC1 was detected in the miR-451 mimic group (Figure 3B). However, this effect was blocked when the binding site was mutated (Figure 3B). Furthermore, we investigated the regulatory role of miR-451 in TSC1 expression. The results showed that the mRNA level of TSC1 was profoundly decreased by the miR-451 mimic but increased by the miR-451 inhibitor (Figure 3C). In a concordant finding, a similar pattern was observed in TSC1 protein expression (Figure 3D). In summary, the data proved that miR-451 downregulated its expression by targeting TSC1.
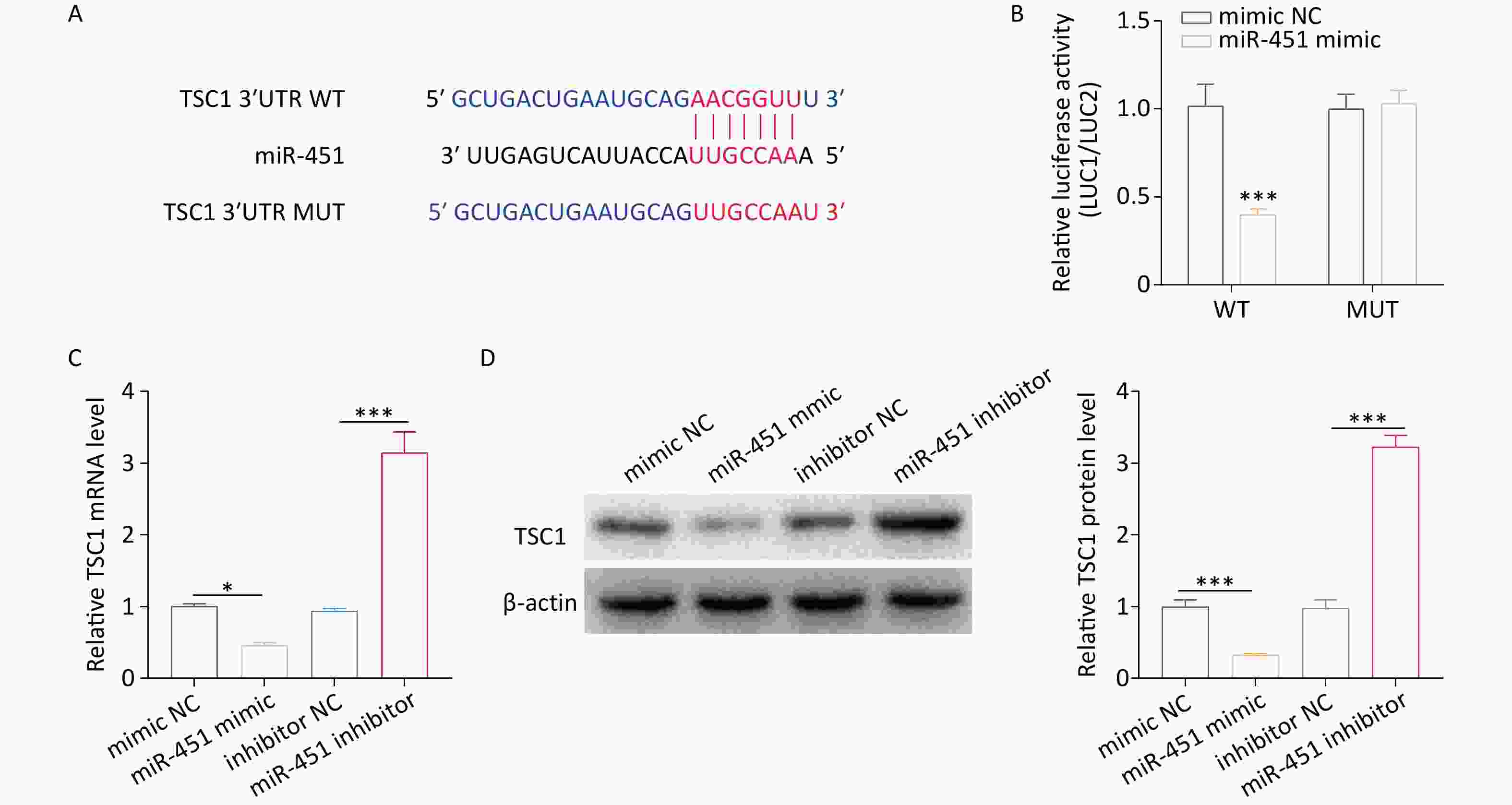
Figure 3. miR-451 decreases its level by directly targeting TSC1. (A) TargetScan was applied for predicting the potential bind sites of miR-451 in TSC1 3’UTR region. (B) The luciferase activity of TSC1 was tested using a dual-luciferase assay in HEK293T cells co-transfected with the miR-451 mimic and the indicated reporter plasmids of TSC1. (C–D) The mRNA and protein expression of TSC1 were detected by qRT-PCR and western blotting, respectively, when alveolar macrophages were transfected with the miR-451 mimic or inhibitor. All experiments were performed in triplicate (*P < 0.05; ***P < 0.001). NC, negative control.
-
TSC1 activates autophagy by inhibiting mTOR signaling pathway. Considering that miR-451 decreases TSC1, it was speculated that miR-451 from hUC-MSC-Exos might suppress autophagy by activating the mTOR pathway and reducing TSC1. To verify this hypothesis, we first determined the effect of hUC-MSC-derived exosomal miR-451 on TSC1 expression and mTOR activation. TSC1 expression was elevated in alveolar macrophages after LPS exposure; however, this elevation was inhibited by treatment with hUC-MSC exosomes (Figure 4A–C). Further analysis suggested that the miR-451 inhibitor reversed the TSC1 decrease caused by hUC-MSC-Exos (Figure 4A–C), showing that miR-451 from hUC-MSC-Exos reduced TSC1 in alveolar macrophages. Conversely, hUC-MSC exosomes increased the levels of p-mTOR/mTOR, which were abolished when miR-451 was inhibited (Figure 4B and C). To verify the role of TSC1 in mTOR signaling and autophagy, TSC1 was knocked down in alveolar macrophages. The mRNA silencing efficiency of si-TSC1-1 was the highest and was used for follow-up experiments (Figure 4D). TSC1 deficiency enhanced p-mTOR/mTOR levels and diminished the expression of ATG13, p-ULK1/ULK1, Beclin-1, and LC3-II/LC3-I, which were reversed by the miR-451 inhibitor, indicating that miR-451 could regulate autophagy via TSC1 (Figure 4E and F). To confirm this finding, GFP-LC3 immunofluorescence staining was performed to examine autophagy. Consistent with the western blot results, TSC1 silencing restrained alveolar macrophage autophagy, which was rescued after exposure to hUC-MSC-Exos containing the miR-451 inhibitor (Figure 4G and H). Therefore, hUC-MSC exosomal miR-451 weakened autophagy via the TSC1/mTOR pathway in alveolar macrophages.
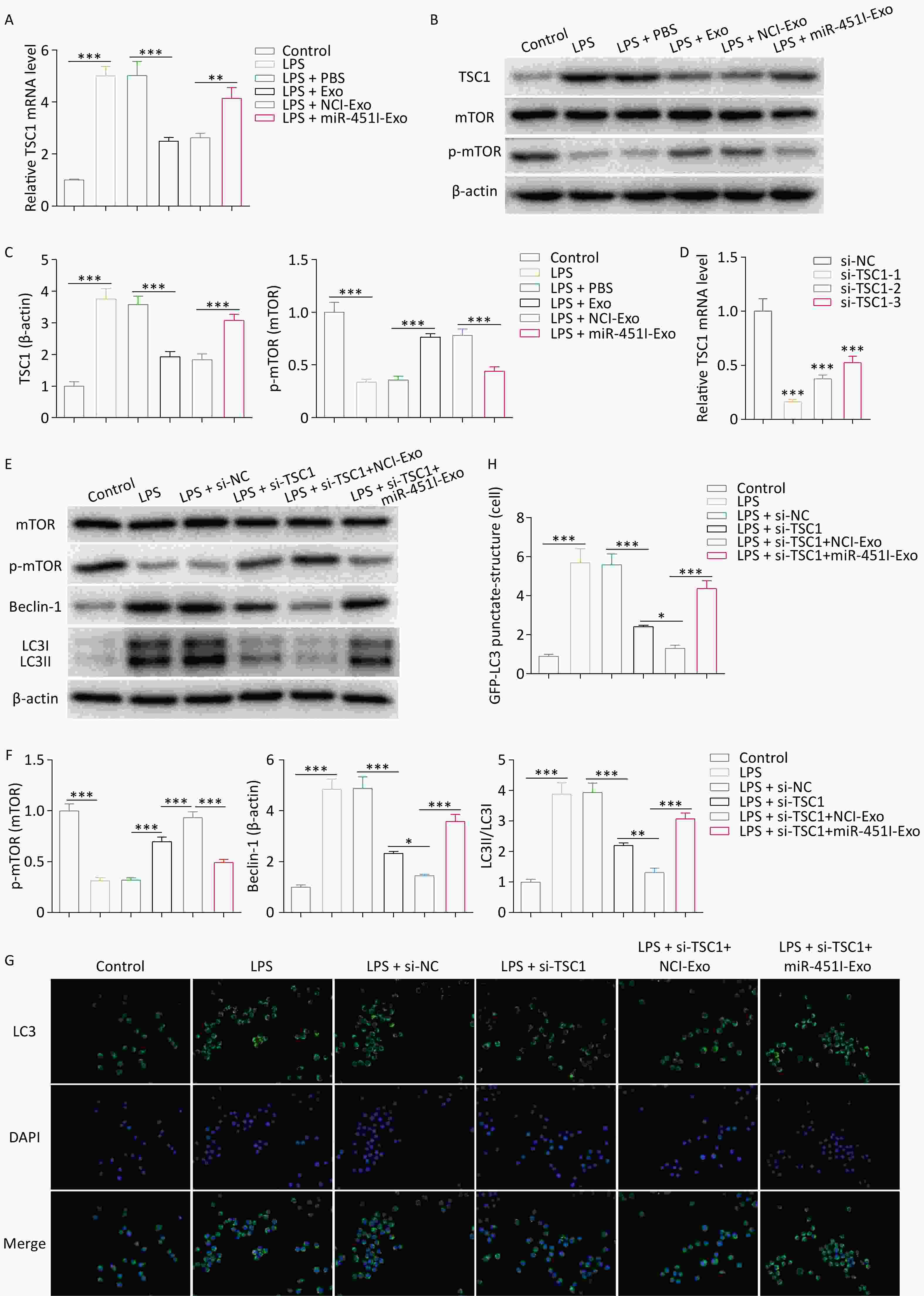
Figure 4. hUC-MSCs exosome miR-451 inhibits alveolar macrophages autophagy through TSC1/mTOR signaling pathway. (A) The mRNA expression of TSC1 was examined by qRT-PCR after alveolar macrophages were treated with LPS and different hUC-MSC-Exos. (B–C) Western blotting was performed to determine the protein expression of TSC1, mTOR, and p-mTOR in alveolar macrophages. (D) qRT-PCR was performed to determine the efficiency of TSC1 silencing in alveolar macrophages. (E–F) Western blotting was conducted to determine the protein expression of ATG13, ULK1, p-ULK1, mTOR, p-mTOR, Beclin-1, and LC3-II/LC3-I to evaluate the effect of TSC1 on autophagy. (G–H) The effect of TSC1 on alveolar macrophage autophagy was examined by GFP-LC3 immunofluorescence staining. All experiments were performed in triplicate (*P < 0.05, **P < 0.01, ***P < 0.001). LPS, lipopolysaccharide; hUC-MSC-Exo, human umbilical cordmesenchymal stem cell-derived exosomes; PBS, phosphate-buffered saline; miR-451I-Exo, exosomes transfected withan miR-451 inhibitor; TSC1, tuberous sclerosis complex 1; mTOR, mammalian target of rapamycin; p-mTOR, Phospho mammalian target of rapamycin; mRNA, messenger RNA; qRT-PCR, quantitative real-time PCR; ATG13, Autophagy Related 13; ULK1, UNC-51-like kinase 1; p-ULK1, Phospho UNC-51-like kinase 1; LC3, microtubule-associated-proteinlight-chain-3.
-
Given that the loss of TSC1 leads to impaired autophagy due to activation of the mTOR pathway, we investigated whether inhibiting mTOR could restore autophagy in si-TSC1 alveolar macrophages. Western blotting revealed that rapamycin treatment suppressed p-mTOR activity in si-TSC1 alveolar macrophages and enhanced autophagy (Figure 5A). Furthermore, this outcome was validated by GFP-LC3 immunofluorescence staining (Figure 5B), which indicated that rapamycin restores autophagy in si-TSC1 alveolar macrophages. To further investigate the contribution of autophagy to the development of in vivo ALI in the absence of TSC1, SD rats were injected with rapamycin 3 days before ALI modeling. Rapamycin partially restored the decreased autophagy levels in burn-induced ALI rats while reducing p-mTOR activity (Figure 5C). Notably, rapamycin increased inflammatory cell infiltration and injury in rats with burn-induced ALI treated with si-TSC1 (Figure 5D). Overall, these results indicate that autophagy induced by deactivation of mTOR signaling exacerbates burn-induced ALI in si-TSC1 rats.
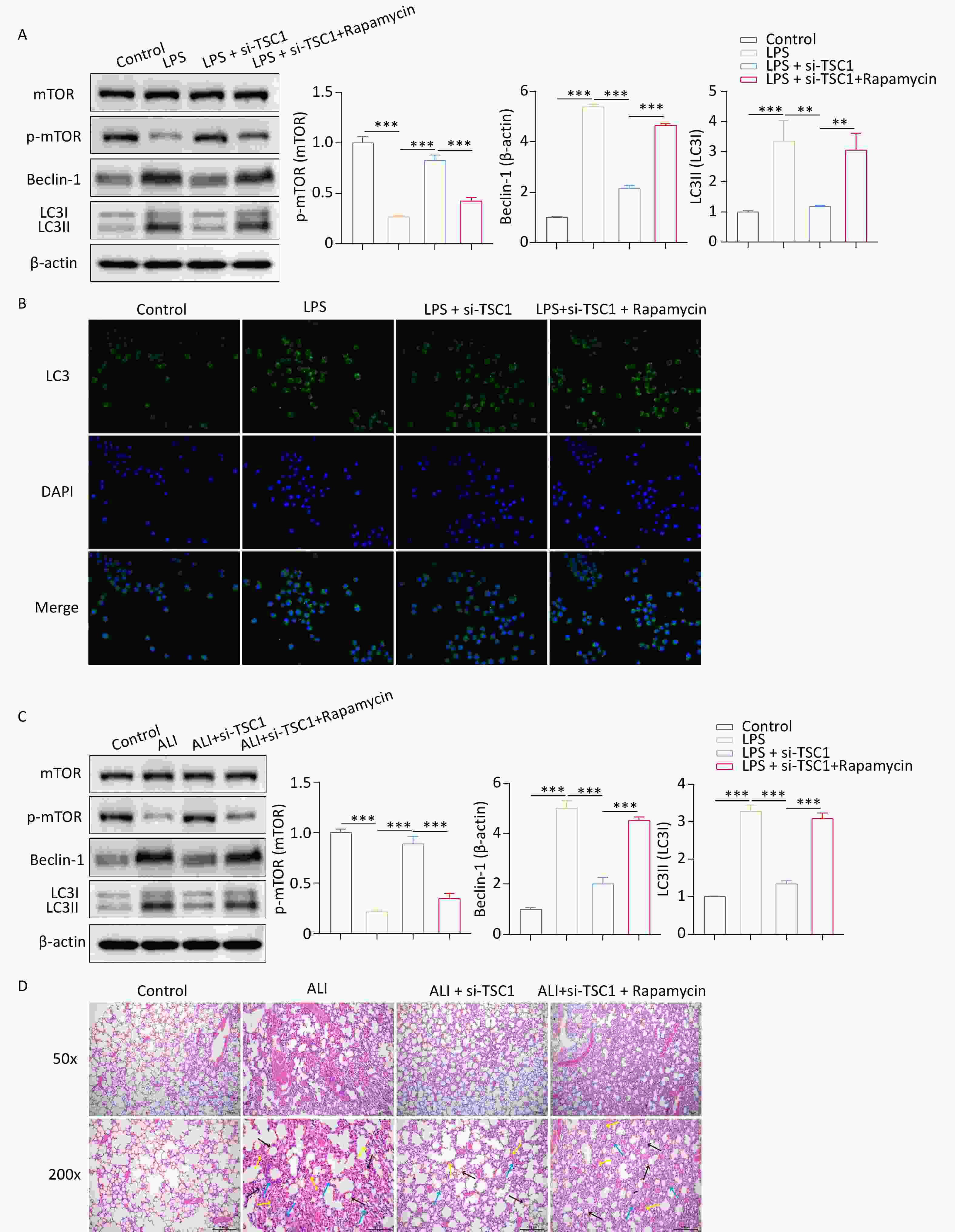
Figure 5. mTOR inhibition potentiates alveolar macrophages autophagy to promote ALI progression. (A) Western blotting was conducted to determine the protein expression of mTOR, p-mTOR, Beclin-1 and LC3-II/LC3-I to evaluate the effect of rapamycin on autophagy. (B) The effect of rapamycin on alveolar macrophage autophagy was examined by GFP-LC3 immunofluorescence staining. (C) The protein expression levels of mTOR, p-mTOR, Beclin-1 and LC3-II/LC3-I in alveolar macrophages from burned rats were tested by western blotting after rapamycin treatment. (D) Hematoxylin and eosin (HE) staining was used to evaluate inflammatory cell infiltration and injury in rapamycin-treated rats with burns. Black arrows indicate interstitial lung swelling, yellow arrows indicate erythrocyte exudation, and blue arrows indicate inflammatory cell infiltration. All experiments were performed in triplicate (**P < 0.01; ***P < 0.001). ALI, acute lung injury; LPS, lipopolysaccharide.
-
Severe burns often result in fatal complications, with ALI being one of the most common and destructive outcomes. The mortality rate for patients with ALI is high, especially those with a burn area of > 30% TBSA[32]; this is often used to establish full-thickness burn models[7]. In this study, we constructed the above models using SD rats to perform in vivo experiments. Consistent with previous studies[21,33], severe burns induced lung injury, manifesting as an elevated secretion of inflammatory cytokines and infiltration of inflammatory cells. ALI is characterized by uncontrolled inflammatory reactions. Controlling inflammation is a major focus in ALI therapy[34]. Recent studies reveal that hUC-MSCs exert strong anti-inflammatory and lung-protective effects[35,36]. Therefore, exploring the functions of hUC-MSCs is crucial. Several studies have demonstrated that hUC-MSCs ameliorate ALI by releasing exosomal molecules such as miRNAs[37,38]. Our previous experiments indicated the role of exosomal miR-451 from hUC-MSCs in ALI improvement[21,24], but the underlying mechanism remain unclear. After confirming these results in the present study, we focused on understanding how miR-451 from hUC-MSC-Exos attenuates ALI.
Autophagy is a highly conserved process by which cytoplasmic components are transported to lysozymes for degradation into primary components to maintain cellular homeostasis. The role of autophagy in ALI pathogenesis has attracted increasing attention. For example, hydrogen sulfide ameliorates ALI by suppressing autophagic activity[39]. MiR-34a may inhibit excessive autophagy to alleviate LPS-induced ALI[40]. The autophagy inhibitor, 3-methyladenine, exhibits protective and therapeutic potential against ALI[41]. Thus, the inhibition of autophagy is beneficial for ALI treatment. Given that macrophages are critical immune cells in the pulmonary inflammatory microenvironment and that macrophage autophagy plays an important role in the regulation of ALI[42], we evaluated whether hUC-MSC exosome miR-451 modulated alveolar macrophage autophagy. Autophagy levels in alveolar macrophages increased after burns, and hUC-MSC exosomes suppressed this increase. Furthermore, the miR-451 inhibitor reversed the autophagy reduction caused by hUC-MSC-derived exosomes, suggesting that miR-451 from hUC-MSC-Exos might exhibit protective effects against ALI by inhibiting overactive macrophage autophagy. Nevertheless, other studies illustrate that suppression of macrophage autophagy aggravates the inflammatory reaction and lung injury[43,44]. Autophagy is a double-edged sword in ALI[42]. Moderate autophagy is beneficial, whereas excessive autophagy causes cell death[45]. Studies have indicated that enhanced autophagy may promote inflammatory responses by modulating the activation of NLRP3 inflammasomes[46], increasing the release of cytokines[47], and regulating the generation of reactive oxygen species[48,49]. Autophagy may play a crucial role in burn-induced ALI. Following burns, cells experience severe stress, leading to increased oxidative stress and inflammatory responses[50,51], ultimately resulting in cell and lung damage. Autophagy mitigates cellular damage by eliminating damaged organelles and proteins, thereby reducing the extent of cell injury and protecting cells from further damage. In summary, targeting autophagy is a promising therapeutic strategy for ALI and its roles and mechanisms should be comprehensively investigated.
It has been proved that molecules carried by exosomes can be transferred to adjacent or other cells, thereby regulating cellular signals[52]. To elucidate whether exosomal miR-451 could be transferred from hUC-MSCs to alveolar macrophages, an in vitro cell model was established using LPS treatment. LPS are often used to induce ALI[53]. After exposure to LPS and exosomes, miR-451 was upregulated in alveolar macrophages; however, miR-451 was downregulated in alveolar macrophages when exosomes were treated with an miR-451 inhibitor. Furthermore, DiI staining validated the phagocytosis of exosomes by alveolar macrophages. These data indicate that exosomal miR-451 may be delivered to alveolar macrophages. Consistent with in vivo results, in vitro study confirmed that miR-451 is a negative regulator of autophagy. This study investigated how miR-451 regulates autophagy in alveolar macrophages. TSC1 has been reported to be a target of miR-451[29]. We also observed that miR-451 downregulates TSC1 expression by directly targeting it. Moreover, LPS-induced TSC1 expression was reduced by hUC-MSC exosomes, but was abolished when exosomes were transfected with the miR-451 inhibitor. These results revealed that in alveolar macrophages, TSC1 expression was reduced by the delivery of miR-451 from hUC-MSC exosomes. It has been documented that miR-451 knockdown promotes autophagy and inhibits mTOR signals[54]. In gastric cancer, exosome-loaded miR-451 is redistributed from tumor cells to T cells and enhances mTOR activity[55]. Conversely, miR-451 suppresses the mTOR pathway in glioma[56]. miR-451a reduces mTOR levels in small cell lung cancer[57]. Notably, in breast cancer, miR-451a lowers p-mTOR activation; however, autophagy is augmented[58]. Based on the above evidence, miR-451 can modulate mTOR signaling, and its regulatory effect on autophagy may depend on the mTOR pathway. However, whether miR-451 regulates autophagy through mTOR signaling in ALI remains unclear. In this study, we verified that hUC-MSC exosome miR-451 activates the mTOR signaling pathway in ALI, thereby reducing autophagy. Finally, we examined the role of TSC1 in miR-451-mediated mTOR activation. Numerous studies have demonstrated that TSC1 enhances autophagy by repressing mTOR pathway[59,60]. The present study showed that silencing TSC1 activated mTOR signaling and reduced alveolar macrophage autophagy. Further analysis revealed that the regulatory effect of miR-451 from hUC-MSC exosomes on autophagy was dependent on the TSC1/mTOR pathway.
Our study validated that exosomal miR-451 from hUC-MSCs attenuated the inflammatory response in ALI. Mechanistically, exosome-enveloped miR-451 was transferred from hUC-MSCs to alveolar macrophages and enhanced autophagy. Furthermore, TSC1 was found to be a direct target of miR-451. miR-451 from hUC-MSCs-Exo inhibits TSC1 and subsequently activates the mTOR signaling pathway to restrain alveolar macrophage autophagy, thereby improving ALI. Collectively, we revealed that miR-451 from hUC-MSC exosomes alleviate ALI by suppressing alveolar macrophage autophagy via the TSC1/mTOR pathway.
This discovery not only broadens the utilization of miR-451 in disease management but also introduces novel ideas and strategies for treating ALI. By conducting comprehensive research on the mechanism by which miR-451 regulates the TSC1/mTOR pathway, a groundwork can be established for the advancement of more accurate ALI therapies. Furthermore, these results offer a robust experimental foundation for considering miR-451 as a therapeutic target for individuals with ALI and serve as a significant point of reference for future clinical translational studies. Moreover, investigating the role of the exosomal miR-451 pathway in stem cell-mediated therapy could offer theoretical support for enhancing the efficacy of stem cell therapeutic approaches. Overall, this study strongly supports the potential of miR-451 as a therapeutic target for ALI, expands the application prospects of stem cell-derived exosomes in inflammatory diseases, and suggests new avenues for future research and development in disease treatments and stem cell therapies.
However, this study had certain limitations. First, our investigation specifically targeted burn-induced ALI and neglected other forms of acute lung injury, potentially limiting the applicability of these findings. Second, while we have proposed the inhibition of alveolar macrophage autophagy by miR-451 from hUC-MSC exosomes, additional research and elucidation are imperative. Although our study suggests the involvement of the TSC1/mTOR pathway, a more comprehensive understanding of its precise molecular mechanism and regulatory network requires further exploration and validation.
-
The authors declare no competing interests.
doi: 10.3967/bes2024.128
MicroRNA-451 from Human Umbilical Cord-Derived Mesenchymal Stem Cell Exosomes Inhibits Alveolar Macrophage Autophagy via Tuberous Sclerosis Complex 1/Mammalian Target of Rapamycin Pathway to Attenuate Burn-Induced Acute Lung Injury in Rats
-
Abstract:
Objective Our previous studies established that microRNA (miR)-451 from human umbilical cord mesenchymal stem cell-derived exosomes (hUC-MSC-Exos) alleviates acute lung injury (ALI). This study aims to elucidate the mechanisms by which miR-451 in hUC-MSC-Exos reduces ALI by modulating macrophage autophagy. Methods Exosomes were isolated from hUC-MSCs. Severe burn-induced ALI rat models were treated with hUC-MSC-Exos carrying the miR-451 inhibitor. Hematoxylin-eosin staining evaluated inflammatory injury. Enzyme-linked immunosorbnent assay measured lipopolysaccharide (LPS), tumor necrosis factor-α, and interleukin-1β levels. qRT-PCR detected miR-451 and tuberous sclerosis complex 1 (TSC1) expressions. The regulatory role of miR-451 on TSC1 was determined using a dual-luciferase reporter system. Western blotting determined TSC1 and proteins related to the mammalian target of rapamycin (mTOR) pathway and autophagy. Immunofluorescence analysis was conducted to examine exosomes phagocytosis in alveolar macrophages and autophagy level. Results hUC-MSC-Exos with miR-451 inhibitor reduced burn-induced ALI and promoted macrophage autophagy. MiR-451 could be transferred from hUC-MSCs to alveolar macrophages via exosomes and directly targeted TSC1. Inhibiting miR-451 in hUC-MSC-Exos elevated TSC1 expression and inactivated the mTOR pathway in alveolar macrophages. Silencing TSC1 activated mTOR signaling and inhibited autophagy, while TSC1 knockdown reversed the autophagy from the miR-451 inhibitor-induced. Conclusion miR-451 from hUC-MSC exosomes improves ALI by suppressing alveolar macrophage autophagy through modulation of the TSC1/mTOR pathway, providing a potential therapeutic strategy for ALI. -
Key words:
- Acute lung injury /
- Human umbilical cord mesenchymal stem cell-derived exosomes /
- MicroRNA-451 /
- Tuberous sclerosis complex 1 /
- Mammalian target of rapamycin pathway /
- Autophagy
&These authors contributed equally to this work.
注释:1) AUTHOR STATEMENT: -
S1. Characterization of hUCMSC-Exos. A, The ultrastructure of hUCMSC-Exo was analyzed by transmission electron microscopy. Scale bar = 100 nm. B, Measurement of hUCMSC-Exos size distribution by Nanoparticle Tracking Analysis. C, Expression of the protein markers CD9 and CD63, which are commonly found in exosomes, was determined by western blot.
Figure 1. miR-451 from hUC-MSCs-Exo improves ALI through suppressing autophagy in alveolar macrophages. (A) Level of miR-451 in hUC-MSC-Exos was detected using qRT-PCR after treatment with the miR-451 inhibitor. (B) We established a rat burn model to simulate ALI in vivo. (C–D) ELISA was performed to examine the contents of LPS, TNF-α and IL-1β in lung tissues and alveolar lavage fluid of burn rats from different treatments. Hematoxylin and eosin staining was used to evaluate inflammatory cell infiltration and injury. Black arrows indicate interstitial lung swelling, yellow arrows indicate erythrocyte exudation, and blue arrows indicate inflammatory cell infiltration. (F) Protein expression of Beclin-1 and LC3-II/LC3-I in alveolar macrophages from rats with burns was tested using western blotting. All experiments were performed in triplicate (***P < 0.001). ALI, acute lung injury; LPS, lipopolysaccharide; hUC-MSC-Exo, human umbilical cord mesenchymal stem cell-derived exosomes; PBS, phosphate-buffered saline; NCI-Exo, exosomes transfected with a control inhibitor; miR-451I-Exo, exosomes transfected with an miR-451 inhibitor.
Figure 2. Alveolar macrophage autophagy is inhibited by miR-451 transferred by hUC-MSCs-Exo. (A) miR-451 expression in alveolar macrophages after LPS exposure and distinct hUC-MSC-Exo treatments was determined by qRT-PCR. (B) Immunofluorescence staining was performed to label hUC-MSC-Exos using Dil. (C–D) The effects of miR-451 from hUC-MSC-Exos on macrophage autophagy were evaluated using GFP-LC3 immunofluorescence staining. (E) Western blotting was performed to examine the protein levels of Beclin-1 and LC3-II/LC3-I in alveolar macrophages. All experiments were performed in triplicate (***P < 0.001). ALI, acute lung injury; LPS, lipopolysaccharide; hUC-MSC-Exo, human umbilical cord mesenchymal stem cell-derived exosomes; PBS, phosphate-buffered saline; NCI-Exo, exosomes transfected with a control inhibitor; miR-451I-Exo, exosomes transfected withan miR-451 inhibitor.
Figure 3. miR-451 decreases its level by directly targeting TSC1. (A) TargetScan was applied for predicting the potential bind sites of miR-451 in TSC1 3’UTR region. (B) The luciferase activity of TSC1 was tested using a dual-luciferase assay in HEK293T cells co-transfected with the miR-451 mimic and the indicated reporter plasmids of TSC1. (C–D) The mRNA and protein expression of TSC1 were detected by qRT-PCR and western blotting, respectively, when alveolar macrophages were transfected with the miR-451 mimic or inhibitor. All experiments were performed in triplicate (*P < 0.05; ***P < 0.001). NC, negative control.
Figure 4. hUC-MSCs exosome miR-451 inhibits alveolar macrophages autophagy through TSC1/mTOR signaling pathway. (A) The mRNA expression of TSC1 was examined by qRT-PCR after alveolar macrophages were treated with LPS and different hUC-MSC-Exos. (B–C) Western blotting was performed to determine the protein expression of TSC1, mTOR, and p-mTOR in alveolar macrophages. (D) qRT-PCR was performed to determine the efficiency of TSC1 silencing in alveolar macrophages. (E–F) Western blotting was conducted to determine the protein expression of ATG13, ULK1, p-ULK1, mTOR, p-mTOR, Beclin-1, and LC3-II/LC3-I to evaluate the effect of TSC1 on autophagy. (G–H) The effect of TSC1 on alveolar macrophage autophagy was examined by GFP-LC3 immunofluorescence staining. All experiments were performed in triplicate (*P < 0.05, **P < 0.01, ***P < 0.001). LPS, lipopolysaccharide; hUC-MSC-Exo, human umbilical cordmesenchymal stem cell-derived exosomes; PBS, phosphate-buffered saline; miR-451I-Exo, exosomes transfected withan miR-451 inhibitor; TSC1, tuberous sclerosis complex 1; mTOR, mammalian target of rapamycin; p-mTOR, Phospho mammalian target of rapamycin; mRNA, messenger RNA; qRT-PCR, quantitative real-time PCR; ATG13, Autophagy Related 13; ULK1, UNC-51-like kinase 1; p-ULK1, Phospho UNC-51-like kinase 1; LC3, microtubule-associated-proteinlight-chain-3.
Figure 5. mTOR inhibition potentiates alveolar macrophages autophagy to promote ALI progression. (A) Western blotting was conducted to determine the protein expression of mTOR, p-mTOR, Beclin-1 and LC3-II/LC3-I to evaluate the effect of rapamycin on autophagy. (B) The effect of rapamycin on alveolar macrophage autophagy was examined by GFP-LC3 immunofluorescence staining. (C) The protein expression levels of mTOR, p-mTOR, Beclin-1 and LC3-II/LC3-I in alveolar macrophages from burned rats were tested by western blotting after rapamycin treatment. (D) Hematoxylin and eosin (HE) staining was used to evaluate inflammatory cell infiltration and injury in rapamycin-treated rats with burns. Black arrows indicate interstitial lung swelling, yellow arrows indicate erythrocyte exudation, and blue arrows indicate inflammatory cell infiltration. All experiments were performed in triplicate (**P < 0.01; ***P < 0.001). ALI, acute lung injury; LPS, lipopolysaccharide.
S1. Sequences used in cell transfection
shRNA name Sequences si-TSC1-#1 Sense: GGUGUUGAUCACCAUGCUACC
Antisense: UAGCAUGGUGAUCAACACCAAsi-TSC1-#2 Sense: CGGCUGAUGUUGUUCAGUACC
Antisense: UACUGAACAACAUCAGCCGAGsi-TSC1-#3 Sense: GACAGUAUAGAGAAAGAUAAG
Antisense: UAUCUUUCUCUAUACUGUCUUmiR-451 mimic Sense: AAACCGUUACCAUUACUGAGUU
Antisense: AACUCAGUAAUGGUAACGGUUUmiR-451 inhibitor AACUCAGUAAUGGUAACGGUUU S2. Primer sequences for qRT-PCR
Gene Sequences miR-451-RT 5’- CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAACTCAGT-3’ miR-451 F: 5’-GCCGGAAACCGTTACCATT-3’ URP 5’-CTCAACTGGTGTCGTGGA-3’ U6-RT F: 5’-CTCGCTTCGGCAGCACA-3’
R: 5’-AACGCTTCACGAATTTGCGT-3’TSC1 F: 5’-GTAAACACGTTGGTGGATT-3’
R: 5’-GCTTTGCCTACATACTCATTC-3’β-actin F: 5’-GTCCCTCACCCTCCCAAAAG-3’
R: 5’-GCTGCCTCAACACCTCAACCC-3’ -
[1] Zheng XF, Zhu F, Fang H, et al. Management of combined massive burn and blast injury: a 20-year experience. Burns, 2020; 46, 75−82. doi: 10.1016/j.burns.2018.11.010 [2] Lang TC, Zhao RL, Kim A, et al. A critical update of the assessment and acute management of patients with severe burns. Adv Wound Care (New Rochelle), 2019; 8, 607−33. doi: 10.1089/wound.2019.0963 [3] Huang CT, Lin HH, Ruan SY, et al. Efficacy and adverse events of high-frequency oscillatory ventilation in adult patients with acute respiratory distress syndrome: a meta-analysis. Crit Care, 2014; 18, R102. doi: 10.1186/cc13880 [4] Hughes KT, Beasley MB. Pulmonary manifestations of acute lung injury: more than just diffuse alveolar damage. Arch Pathol Lab Med, 2017; 141, 916−22. doi: 10.5858/arpa.2016-0342-RA [5] Mowery NT, Terzian WTH, Nelson AC. Acute lung injury. Curr Probl Surg, 2020; 57, 100777. doi: 10.1016/j.cpsurg.2020.100777 [6] D'Alessio FR. Mouse models of acute lung injury and ARDS. In: Alper S, Janssen WJ. Lung Innate Immunity and Inflammation. Humana Press. 2018, 341-50. [7] Cai WX, Shen K, Ji P, et al. The Notch pathway attenuates burn-induced acute lung injury in rats by repressing reactive oxygen species. Burns Trauma, 2022; 10, tkac008. doi: 10.1093/burnst/tkac008 [8] Song D, Zhao M, Feng LX, et al. Salidroside attenuates acute lung injury via inhibition of inflammatory cytokine production. Biomed Pharmacother, 2021; 142, 111949. doi: 10.1016/j.biopha.2021.111949 [9] Lei JL, Wei YL, Song PC, et al. Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress. Eur J Pharmacol, 2018; 818, 110−4. doi: 10.1016/j.ejphar.2017.10.029 [10] Xu YP, Zhu JQ, Feng B, et al. Immunosuppressive effect of mesenchymal stem cells on lung and gut CD8+ T cells in lipopolysaccharide-induced acute lung injury in mice. Cell Prolif, 2021; 54, e13028. doi: 10.1111/cpr.13028 [11] Xu F, Hu Y, Zhou JB, et al. Mesenchymal stem cells in acute lung injury: are they ready for translational medicine? J Cell Mol Med, 2013; 17, 927-35. [12] Monsel A, Zhu YG, Gudapati V, et al. Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases. Expert Opin Biol Ther, 2016; 16, 859−71. doi: 10.1517/14712598.2016.1170804 [13] Guiot J, Struman I, Louis E, et al. Exosomal miRNAs in lung diseases: from biologic function to therapeutic targets. J Clin Med, 2019; 8, 1345. doi: 10.3390/jcm8091345 [14] Mizuta Y, Akahoshi T, Guo J, et al. Exosomes from adipose tissue-derived mesenchymal stem cells ameliorate histone-induced acute lung injury by activating the PI3K/Akt pathway in endothelial cells. Stem Cell Res Ther, 2020; 11, 508. doi: 10.1186/s13287-020-02015-9 [15] He CJ, Zheng S, Luo Y, et al. Exosome theranostics: biology and translational medicine. Theranostics, 2018; 8, 237−55. doi: 10.7150/thno.21945 [16] Yi XM, Wei XX, Lv HJ, et al. Corrigendum to "Exosomes derived from microRNA-30b-3p-overexpressing mesenchymal stem cells protect against lipopolysaccharide-induced acute lung injury by inhibiting SAA3" [Exp. Cell. Res. 383 (2019) 111, 454]. Exp Cell Res, 2020; 394, 112160. doi: 10.1016/j.yexcr.2020.112160 [17] Wei XX, Yi XM, Lv HJ, et al. Correction: MicroRNA-377-3p released by mesenchymal stem cell exosomes ameliorates lipopolysaccharide-induced acute lung injury by targeting RPTOR to induce autophagy. Cell Death Dis, 2020; 11, 746. doi: 10.1038/s41419-020-02976-y [18] Xu Q, Huang GD, Duan GC, et al. MicroRNA-147b alleviates inflammation and apoptosis in acute lung injury via inhibition of p38 MAPK signaling pathway. Eur Rev Med Pharmacol Sci, 2021; 25, 1974−81. [19] Zhang XL, An J, Deng YZ, et al. A novel miRNA-762/NFIX pathway modulates LPS-induced acute lung injury. Int Immunopharmacol, 2021; 100, 108066. doi: 10.1016/j.intimp.2021.108066 [20] Ahmad S, Zaki A, Manda K, et al. Vitamin-D ameliorates sepsis-induced acute lung injury via augmenting miR-149-5p and downregulating ER stress. J Nutr Biochem, 2022; 110, 109130. doi: 10.1016/j.jnutbio.2022.109130 [21] Liu JS, Du J, Cheng X, et al. Exosomal miR-451 from human umbilical cord mesenchymal stem cells attenuates burn-induced acute lung injury. J Chin Med Assoc, 2019; 82, 895−901. doi: 10.1097/JCMA.0000000000000189 [22] Huang XF, Xiu HQ, Zhang SF, et al. The role of macrophages in the pathogenesis of ALI/ARDS. Mediators Inflamm, 2018; 2018, 1264913. [23] Deng JF, He ZP, Li XR, et al. Huangkui capsule attenuates lipopolysaccharide-induced acute lung injury and macrophage activation by suppressing inflammation and oxidative stress in mice. Evid Based Complement Alternat Med, 2021; 2021, 6626483. [24] Liu JS, Xing FX, Fu QY, et al. hUC-MSCs exosomal miR-451 alleviated acute lung injury by modulating macrophage M2 polarization via regulating MIF-PI3K-AKT signaling pathway. Environ Toxicol, 2022; 37, 2819−31. doi: 10.1002/tox.23639 [25] Hu Y, Liu J, Wu YF, et al. mTOR and autophagy in regulation of acute lung injury: a review and perspective. Microbes Infect, 2014; 16, 727−34. doi: 10.1016/j.micinf.2014.07.005 [26] Fan EKY, Fan J. Regulation of alveolar macrophage death in acute lung inflammation. Respir Res, 2018; 19, 50. doi: 10.1186/s12931-018-0756-5 [27] Hu R, Chen ZF, Yan J, et al. Complement C5a exacerbates acute lung injury induced through autophagy-mediated alveolar macrophage apoptosis. Cell Death Dis, 2014; 5, e1330. doi: 10.1038/cddis.2014.274 [28] Qiu P, Liu Y, Chen KY, et al. Hydrogen-rich saline regulates the polarization and apoptosis of alveolar macrophages and attenuates lung injury via suppression of autophagy in septic rats. Ann Transl Med, 2021; 9, 974. doi: 10.21037/atm-21-2489 [29] Song L, Su M, Wang SY, et al. MiR-451 is decreased in hypertrophic cardiomyopathy and regulates autophagy by targeting TSC1. J Cell Mol Med, 2014; 18, 2266−74. doi: 10.1111/jcmm.12380 [30] Zhang HB, Cicchetti G, Onda H, et al. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest, 2003; 112, 1223−33. doi: 10.1172/JCI200317222 [31] TargetScan Human. https: //www. targetscan. org/. [2023-11-8]. [32] Liu JY, Liu JL, Wang H, et al. Protective effect of celastrol for burn-induced acute lung injury in rats. Int J Clin Exp Pathol, 2019; 12, 576−83. [33] Xiao MJ, Zou XF, Li B, et al. Simulated aeromedical evacuation exacerbates burn induced lung injury: targeting mitochondrial DNA for reversal. Mil Med Res, 2021; 8, 30. [34] Luo JH, Zhan JH, You HY, et al. MicroRNA-146a/Toll-like receptor 4 signaling protects against severe burn-induced remote acute lung injury in rats via anti-inflammation. Mol Med Rep, 2018; 17, 8377−84. [35] Hu XH, Liu LY, Wang Y, et al. Human umbilical cord-derived mesenchymal stem cells alleviate acute lung injury caused by severe burn via secreting TSG-6 and inhibiting inflammatory response. Stem Cells Int, 2022; 2022, 8661689. [36] Liu LY, Song HF, Duan HJ, et al. TSG-6 secreted by human umbilical cord-MSCs attenuates severe burn-induced excessive inflammation via inhibiting activations of P38 and JNK signaling. Sci Rep, 2016; 6, 30121. doi: 10.1038/srep30121 [37] Wei XX, Yi XM, Lv HJ, et al. MicroRNA-377-3p released by mesenchymal stem cell exosomes ameliorates lipopolysaccharide-induced acute lung injury by targeting RPTOR to induce autophagy. Cell Death Dis, 2020; 11, 657. doi: 10.1038/s41419-020-02857-4 [38] Zheng YF, Liu JY, Chen P, et al. Retraction notice to "Exosomal miR-22-3p from human umbilical cord blood-derived mesenchymal stem cells protects against lipopolysaccharid-induced acute lung injury" [Life Sci. 269 (2021) 119004]. Life Sci, 2023; 322, 121601. doi: 10.1016/j.lfs.2023.121601 [39] Xu XL, Li H, Gong Y, et al. Hydrogen sulfide ameliorated lipopolysaccharide-induced acute lung injury by inhibiting autophagy through PI3K/Akt/mTOR pathway in mice. Biochem Biophys Res Commun, 2018; 507, 514−8. doi: 10.1016/j.bbrc.2018.11.081 [40] Song L, Zhou FL, Cheng LJ, et al. MicroRNA-34a suppresses autophagy in alveolar type II epithelial cells in acute lung injury by inhibiting FoxO3 expression. Inflammation, 2017; 40, 927−36. doi: 10.1007/s10753-017-0537-1 [41] Slavin SA, Leonard A, Grose V, et al. Autophagy inhibitor 3-methyladenine protects against endothelial cell barrier dysfunction in acute lung injury. Am J Physiol Lung Cell Mol Physiol, 2018; 314, L388−96. doi: 10.1152/ajplung.00555.2016 [42] Liu C, Xiao K, Xie LX. Progress in preclinical studies of macrophage autophagy in the regulation of ALI/ARDS. Front Immunol, 2022; 13, 922702. doi: 10.3389/fimmu.2022.922702 [43] He N, Tan HY, Deng XY, et al. MiR-223-3p-loaded exosomes from bronchoalveolar lavage fluid promote alveolar macrophage autophagy and reduce acute lung injury by inhibiting the expression of STK39. Hum Cell, 2022; 35, 1736−51. doi: 10.1007/s13577-022-00762-w [44] Ying Y, Sun CB, Zhang SQ, et al. Induction of autophagy via the TLR4/NF-κB signaling pathway by astragaloside Ⅳ contributes to the amelioration of inflammation in RAW264.7 cells. Biomed Pharmacother, 2021; 137, 111271. doi: 10.1016/j.biopha.2021.111271 [45] Gump JM, Thorburn A. Autophagy and apoptosis: what is the connection? Trends Cell Biol, 2011; 21, 387-92. [46] Wang LQ, Cai J, Zhao X, et al. Palmitoylation prevents sustained inflammation by limiting NLRP3 inflammasome activation through chaperone-mediated autophagy. Mol Cell, 2023; 83, 281-97. e10. [47] Cao Y, Chen JH, Ren GF, et al. Punicalagin prevents inflammation in LPS-induced RAW264.7 macrophages by inhibiting FoxO3a/autophagy signaling pathway. Nutrients, 2019; 11, 2794. doi: 10.3390/nu11112794 [48] Shaw GC, Cope JJ, Li LT, et al. Mitoferrin is essential for erythroid iron assimilation. Nature, 2006; 440, 96−100. doi: 10.1038/nature04512 [49] Fokam D, Hoskin D. Instrumental role for reactive oxygen species in the inflammatory response. Front Biosci (Landmark Ed), 2020; 25, 1110−19. doi: 10.2741/4848 [50] Guo YX, Liu YR, Zhao SH, et al. Oxidative stress-induced FABP5 S-glutathionylation protects against acute lung injury by suppressing inflammation in macrophages. Nat Commun, 2021; 12, 7094. doi: 10.1038/s41467-021-27428-9 [51] Kong LM, Deng J, Zhou X, et al. Sitagliptin activates the p62-Keap1-Nrf2 signalling pathway to alleviate oxidative stress and excessive autophagy in severe acute pancreatitis-related acute lung injury. Cell Death Dis, 2021; 12, 928. doi: 10.1038/s41419-021-04227-0 [52] Zhang HT, Wang L, Li CY, et al. Exosome-induced regulation in inflammatory bowel disease. Front Immunol, 2019; 10, 1464. doi: 10.3389/fimmu.2019.01464 [53] Li XH, Wei YL, Li SM, et al. Zanubrutinib ameliorates lipopolysaccharide-induced acute lung injury via regulating macrophage polarization. Int Immunopharmacol, 2022; 111, 109138. doi: 10.1016/j.intimp.2022.109138 [54] Luan PX, Chen XM, Zhang XF, et al. Role of miR-451 in mediating cadmium induced head kidney injury in common carp via targeting cacna1ab through autophagy pathways. Aquat Toxicol, 2022; 248, 106201. doi: 10.1016/j.aquatox.2022.106201 [55] Liu F, Bu ZY, Zhao F, et al. Increased T-helper 17 cell differentiation mediated by exosome-mediated microRNA-451 redistribution in gastric cancer infiltrated T cells. Cancer Sci, 2018; 109, 65−73. doi: 10.1111/cas.13429 [56] Nan Y, Guo HB, Guo LY, et al. MiRNA-451 inhibits glioma cell proliferation and invasion through the mTOR/HIF-1α/VEGF signaling pathway by targeting CAB39. Hum Gene Ther Clin Dev, 2018; 29, 156−66. doi: 10.1089/humc.2018.133 [57] Cui JY, Wang J, Shen YY, et al. Suppression of HELLS by miR-451a represses mTOR pathway to hinder aggressiveness of SCLC. Genes Genomics, 2021; 43, 105−14. doi: 10.1007/s13258-020-01028-1 [58] Liu ZR, Song Y, Wan LH, et al. Over-expression of miR-451a can enhance the sensitivity of breast cancer cells to tamoxifen by regulating 14-3-3ζ, estrogen receptor α, and autophagy. Life Sci, 2016; 149, 104−13. doi: 10.1016/j.lfs.2016.02.059 [59] Li CY, Chen YP, Chen XP, et al. Downregulation of MicroRNA-193b-3p promotes autophagy and cell survival by targeting TSC1/mTOR signaling in NSC-34 cells. Front Mol Neurosci, 2017; 10, 160. doi: 10.3389/fnmol.2017.00160 [60] Wang YQ, Zhang XY, Tang W, et al. miR-130a upregulates mTOR pathway by targeting TSC1 and is transactivated by NF-κB in high-grade serous ovarian carcinoma. Cell Death Differ, 2017; 24, 2089−100. doi: 10.1038/cdd.2017.129 -
 23393+Supplementary Materials.pdf
23393+Supplementary Materials.pdf

-




 下载:
下载:





 Quick Links
Quick Links