-
Gas explosions are one of the most lethal types of coal mine safety accidents. The powerful shock waves these explosions produce can cause damage to the lung tissue of nearby individuals, resulting in symptoms including pulmonary hemorrhage and pulmonary edema[1]. At present, there is no effective treatment for gas explosion-induced lung injuries; therefore, there is an urgent need to identify new therapeutic means to improve the clinical management of the system of treatment for lung injury caused by gas explosions. Mesenchymal stem cells (MSCs) are adult stem cells with self-renewal ability and multilineage differentiation potential that exert their therapeutic potential through various mechanisms, including the regulation of inflammation and immune responses[2]. Reports on the therapeutic role of MSCs in blast lung injury remain scarce. Exosomes are extracellular vesicles secreted by most eukaryotic cells. Bone marrow mesenchymal stem cell (BMSC)-derived exosomes have been shown to play an active role in the treatment of lung diseases[3]; however, few studies have been conducted on the role and mechanisms of gas explosions in the recovery from lung injury. The aim of this study was to establish a model of gas explosion-induced lung injury and tail-vein injection of BMSCs and exosomes to evaluate their therapeutic effects, explore the potential mechanisms, and provide a theoretical basis for the treatment of explosive lung injury.
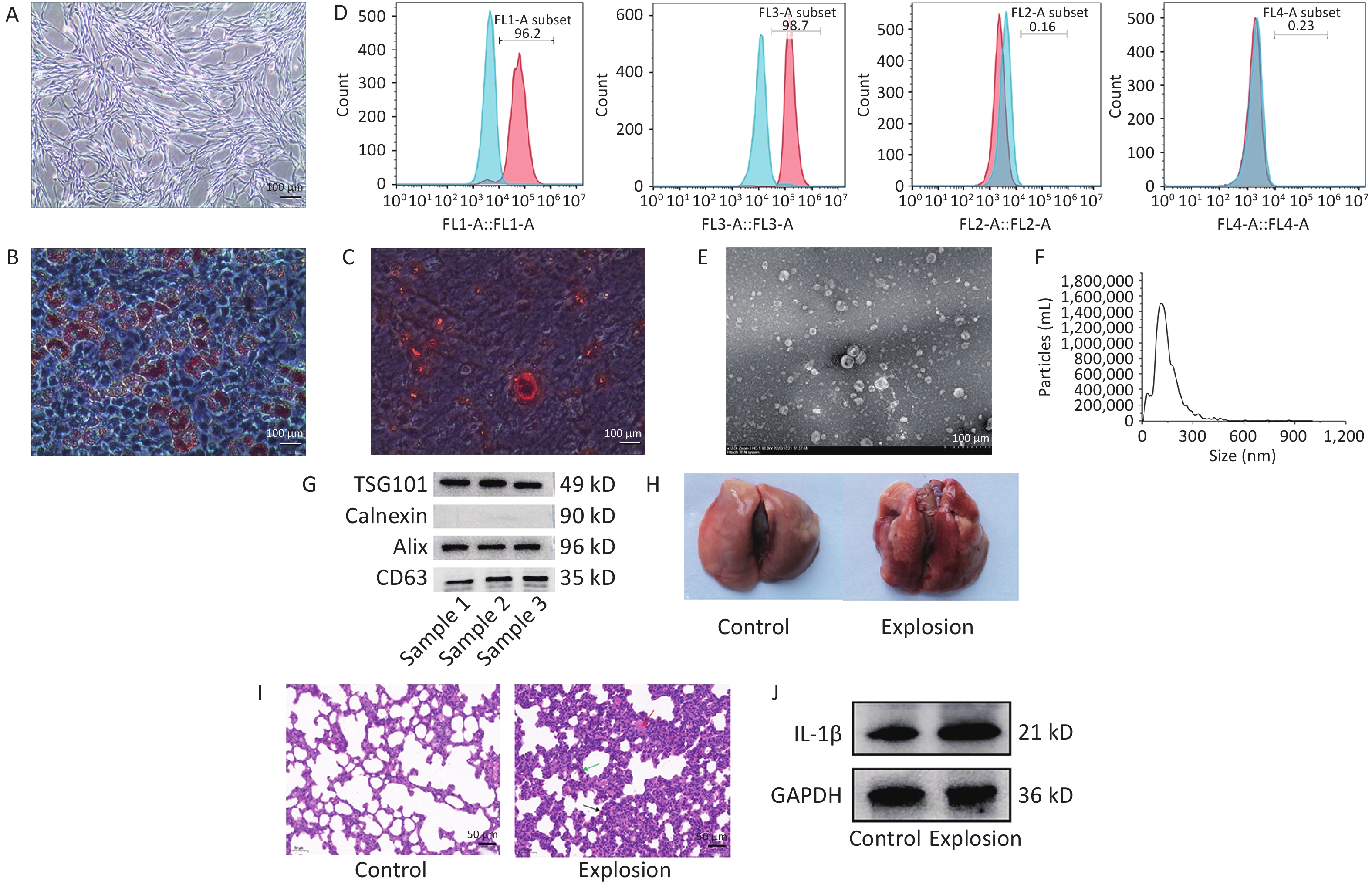
We confirmed the biological characterization and purity of BMSCs for the identification of BMSCs using the following three elements: (i) light microscopy, which was used to investigate the growth morphology of the cells, (ii) adipogenic and osteogenic induction studies, which were used to assess the capacity of the BMSCs to differentiate, and (iii) flow cytometry, which was used to identify the cell surface molecular markers. Under the microscope, the cells converged and swirled together in spindle- or fibroblast-like structures (Figure 1A). Second, lipid droplets of different sizes were observed in adipogenesis-induced BMSCs after Oil Red O staining (Figure 1B), and dark red calcium nodules were observed in osteogenesis-induced BMSCs after alizarin red staining (Figure 1C), indicating that the cultured cells successfully differentiated into adipocytes and osteocytes and had differentiation potential. Finally, using flow cytometry, the expression of positive molecular markers CD90 and CD29 in BMSCs was detected at 96.2% and 98.7%, respectively, and negative molecular markers CD45 and CD11b at 0.16% and 0.23%, respectively, which were lower than 1% (Figure 1D).
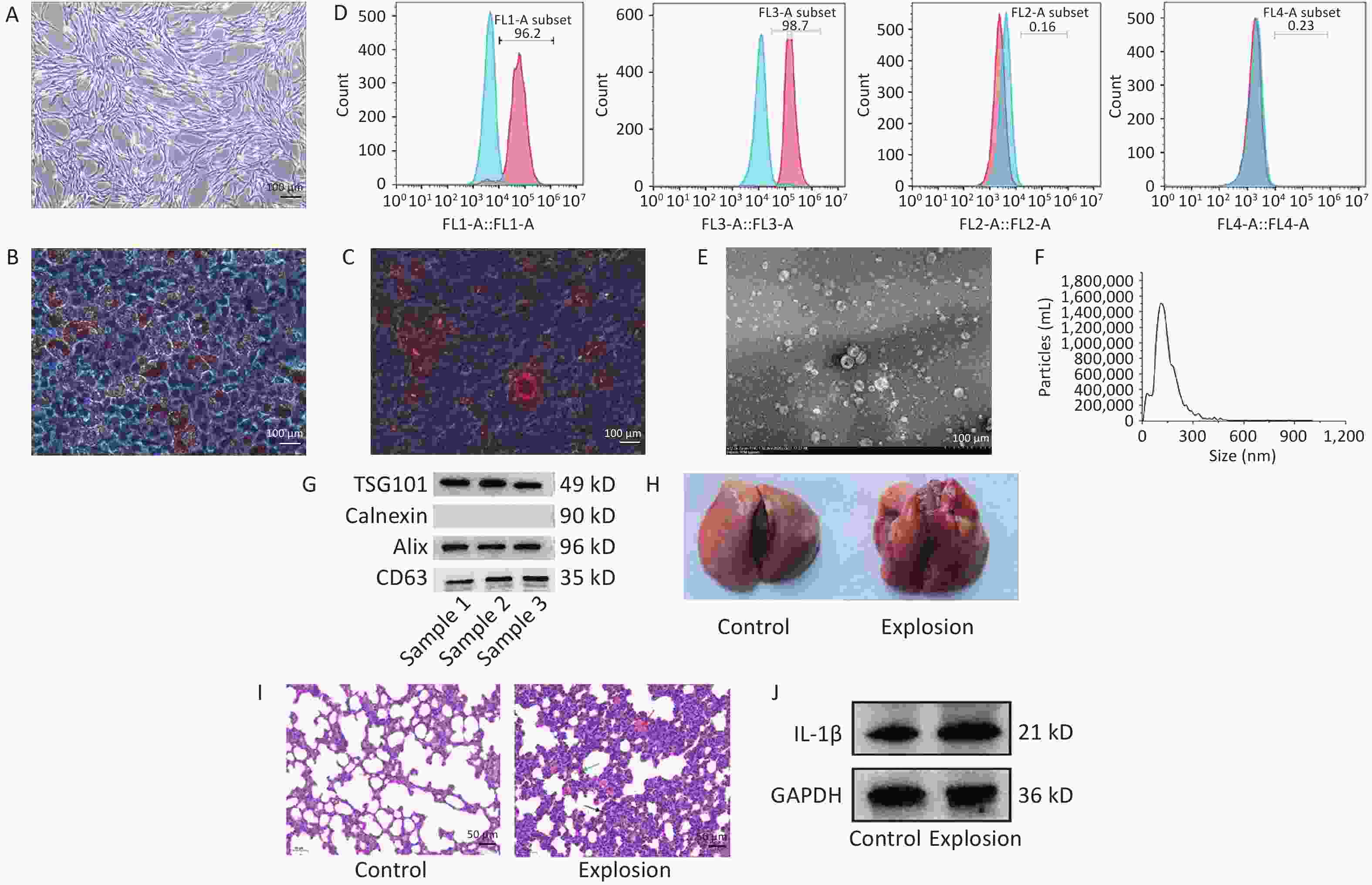
Figure 1. Identification of BMSCs and exosomes and modeling of lung injury in gas explosions. (A) An optical microscope (40×) was used to examine the morphology of passage three BMSCs (n = 3; scale bar = 100 µm). (B) Oil Red staining (200×) was used to demonstrate BMSC adipogenic induction (n = 3; scale bar = 100 µm). (C) Alizarin Red staining (200×) was used to demonstrate osteogenic induction of BMSCs (n = 3; scale bar = 100 µm). (D) Flow cytometry detection of CD90, CD29, CD45, and CD11b (n = 3). (E) Transmission electron microscopy was used to examine the morphology of exosomes (TEM) (n = 3; scale bar = 500 nm). (F) Nanoparticle tracking analysis was used to determine the size distribution of exosomes (NTA) (n = 3). (G) Western blot was used to identify exosome markers such as ALIX, TSG101, Calnexin, and CD63 (n = 3). (H) Rat lungs were examined grossly for pathology 72 hours after the explosion (n = 3). (I) Typical H&E staining of rats’ damaged and functional lungs (black arrow: inflammatory cell infiltration, green arrow: thickening of alveolar septum, red arrow: diffuse hemorrhage; n = 3; scale bar = 50 µm). (J) Western blotting was used to quantify IL-1β protein expression in the control and explosion groups (n = 3).
We collected the culture medium supernatant and extracted the exosomes by ultracentrifugation. The lipid bilayer structure of the exosomes was observed by transmission electron microscopy to resemble that of tea saucers (Figure 1E). The number and size distribution of the particles in the exosomes were examined by Nanoparticle Tracking Analysis (NTA) (Figure 1F), which showed that the average particle size of the exosomes was 127.7 nm, with most particles ranging in size from 30 to 200 nm. The exosome-positive markers ALG-2-interacting protein X (ALIX), CD63, and tumor susceptibility gene 101 (TSG101) were expressed in the particles, according to the western blot data, while calnexin, a negative marker, was not observed (Figure 1G). These findings demonstrate that exosomes were purified from the supernatant of the rat BMSC cultures.
We randomly divided 50 rats into a control group (without any treatment), an explosion group (only gas explosion treatment), a phosphate buffer saline (PBS) solvent control group (within half an hour after gas explosion, 1 mL of PBS injected through the tail vein), a BMSCs group (2 × 106 BMSCs suspended in 1 mL of PBS injected via the tail vein within half an hour after gas explosion), and a BMSC-Exo group (50 µg of exosomes suspended in 1 mL of PBS injected via tail vein within half an hour after gas explosion), with 10 rats in each group. A gas explosion biocide system with a surge tube was used to establish a model of gas explosion-induced lung injury in rats (Supporting Information). The results showed that the lungs of the control rats were red and uniform in color, and the surface of the lobes was smooth; detailed pathological examination of the lungs of the rats after the gas explosion revealed extensive hemorrhaging (Figure 1H). In the explosion group, hematoxylin and eosin (H&E) staining revealed edema, inflammatory cell infiltration, incomplete alveolar septal thickening, and widespread bleeding compared to the control group (Figure 1I). In addition, we detected IL-1β expression, which was significantly increased in the explosion group, indicating a significant inflammatory response (Figure 1J). These findings indicate that the gas explosion-induced blast lung injury model was successfully established.
The lung wet-to-dry (W/D) ratio is another biomarker of lung injury and a direct indicator of pulmonary edema. After the gas explosion, the W/D ratio significantly increased in the explosion and PBS groups. BMSC and BMSC-Exo injections successfully reduced the increase in the lung W/D ratio caused by the gas explosion (Figure 2A). Furthermore, it has been shown that exosomes are effective in improving 7-day survival and reducing lung disease changes and pulmonary vascular permeability in rats with acute lung injury[1,3]. Therefore, next, we determined the protein expression levels of VE-cadherin and ZO-1, important microvascular endothelial adhesion connective proteins. VE-cadherin and ZO-1 were found to be significantly downregulated in both the blast and PBS groups. By contrast, the expression of both ZO-1 and VE-cadherin increased after treatment with BMSCs and BMSCs-Exos (Figure 2B, D, E, G), consistent with the western blotting, PCR, and immunofluorescence results. These findings suggest that BMSCs and BMSCs-Exos can effectively maintain the integrity of the vascular endothelial barrier. Recent studies have shown that BMSCs and their exosomes promote angiogenesis, both in vitro and in vivo[4,5]. Therefore, we also observed the damage and repair status of vascular endothelial cells and found that the expression of the vascular endothelial marker CD31 was significantly decreased and that of the damage marker VWF was increased after the gas explosion, whereas BMSCs and BMSCs-Exos upregulated the expression of CD31 and decreased the expression of VWF (Figure 2C, F, H). These results indicate that BMSCs and BMSCs-Exos effectively reduced vascular endothelial injury and promoted vascular endothelial cell generation.
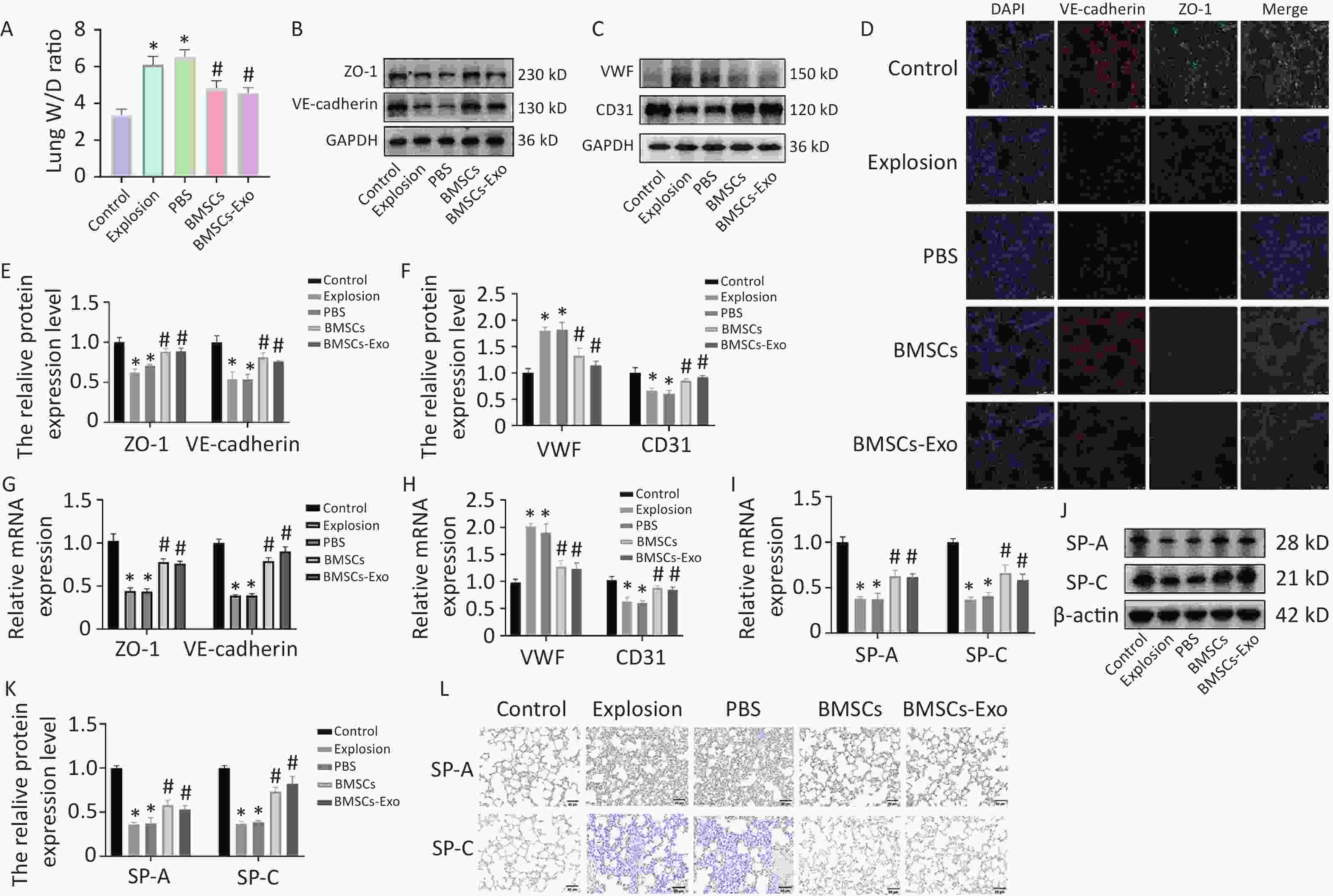
Figure 2. BMSCs and BMSCs-Exo reduce vascular permeability and promote endothelial cell repair while protecting alveolar epithelial cells. (A) Lung W/D ratio in each group 72 hours after the explosion (n = 3). Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (B–C) Western blot results for ZO-1, VE-cadherin, VWF, and CD31 (n = 3). (D) Immunofluorescence results (100×) of ZO-1 and VE-cadherin (n = 3; scale bar = 50 µm). (E–F) Quantitative protein expression of ZO-1, VE-cadherin, VWF, and CD31. Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (G–H) mRNA expression of ZO-1, VE-cadherin, VWF, and CD31 (n = 3). Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (I) mRNA expression of SP-A and SP-C (n = 3). Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (J) Western blot results for SP-A and SP-C (n = 3). (K) Quantitative protein expression of SP-A and SP-C. Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (L) IHC staining for SP-A and SP-C. (200×; n = 3; scale bar = 50 µm).
In a model of idiopathic pulmonary fibrosis, BMSC treatment increased the synthesis of surface-active protein C and improved the repair of alveolar epithelial type II cells[6], while SP-A, an anti-inflammatory molecule, inhibited various potential pro-inflammatory responses[7]. Our results show that the expression of SP-A and SP-C significantly decreased after gas exposure, suggesting a possible inflammatory response with impaired alveolar epithelial cell function. Compared with the explosion group, the expression of SP-A and SP-C was increased in the BMSC and BMSC-Exo groups (Figure 2I–L), suggesting that BMSCs and BMSCs-Exos attenuated the inflammatory response of alveolar epithelial cells and stabilized respiratory membrane function.
Lung tissue was stained with H&E to detect pathological alterations and assess inflammatory infiltration and response. The scoring criteria were as follows: After the sections were fully swept, 10 fields of view were randomly observed and scored for histopathological changes in the lungs, including lung tissue structure and inflammation. The structural damage of the lung tissue was scored in terms of whether the tissue structure was intact, interstitial thickening, or alveolar hemorrhage, whereas inflammation was scored in terms of the aggregation of neutrophils in the alveoli and interstitium. Ten fields of view were averaged to obtain a score for each section[8]. The alveolar structures in the control group were full and distinct. The alveolar structure was disrupted in the gas explosion model group, along with a substantial region of inflammatory cell infiltration and bleeding. After the transplantation of BMSCs and BMSCs-Exos, the overall structure of the alveoli was improved, destruction was markedly reduced, and the aggregation of inflammatory cells was markedly diminished (Figure 3A, B). In addition, pro-inflammatory cytokines, such as TNF-α and Interleukin-1β (IL-1β), play a crucial role in lung inflammation. We measured the levels of these cytokines in the lung tissue by enzyme-linked immunosorbent assay (ELISA) and real time quantitative-polymerase chin reaction (qRT-PCR) and found that these cytokines significantly increased after the gas explosion. However, the injection of BMSCs and BMSCs-Exos blocked the growth of pro-inflammatory factors (Figure 3C–F). IL-10 is an important anti-inflammatory cytokine that can inhibit the inflammatory response and restore the balance between inflammatory and anti-inflammatory responses to play a protective role. Therefore, we also measured the expression level of IL-10 and found that the level of IL-10 decreased significantly after the gas explosion, while the expression of IL-10 increased after the injection of BMSCs and BMSCS-Exo (Figure 3C, G). The ELISA and qRT-PCR results were consistent with these observations. Thus, these results suggest that BMSCs and their exosomes exert anti-inflammatory effects against gas explosion-induced lung injury.
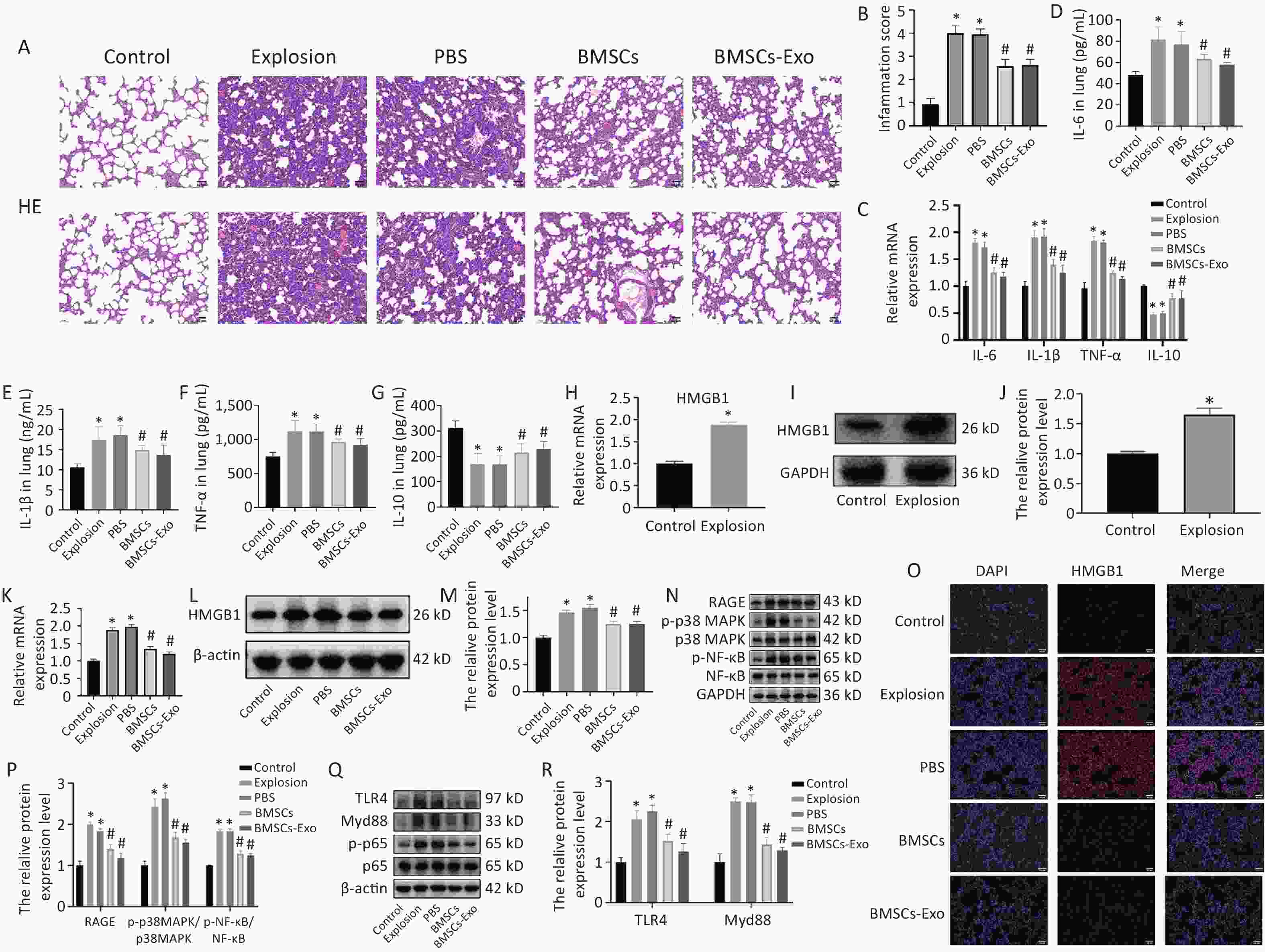
Figure 3. BMSCs and BMSCs-Exo effectively alleviate lung injury and inflammation and influence HMGB1/NF-κB activation. (A) Using H&E staining, lung tissue pathological alterations were observed 72 h after the gas explosion (200×; scale bar = 50 µm). (B) An inflammatory score was used to gauge the degree of lung inflammation (n = 10). Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (C) TNF-α, IL-1β, IL-6, and IL-10 mRNA expression in lung tissue (n = 3). Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (D–G) TNF-α, IL-1β, IL-6, and IL-10 protein expression levels in lung tissue were assessed using ELISA (n = 10). Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (H–J) The amount of HMGB1 expressed in the control and explosion groups (n = 3). Data are presented as the mean ± SD, with *P < 0.05 vs. the control group. (K–M) Western blotting and qRT-PCR were used to evaluate changes in HMGB1 expression (n = 3). Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (O) Immunofluorescence detection (200×) of HMGB1 expression level in each group (n = 3). (N) Western blotting was used to evaluate the expression of RAGE, p38 MAPK, p-p38 MAPK, NF-κB, and p-NF-κB proteins (n = 3). (P) Quantification of RAGE, p-p38 MAPK, and p-NF-κB protein expression in lung tissue. Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (Q) Western blotting was used to determine the expression of TLR4, Myd88 NF-κB, and p-NF-κB proteins (n = 3). (R) Quantification of TLR4 and Myd88a protein expression in lung tissue. Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group.
According to previous studies, high mobility group protein B1 (HMGB1) binds to receptor for advanced glycation end-products (RAGE) and toll-like receptor 4 (TLR4), stimulates the nuclear factor kappa-B (NF-κB) signaling pathway, and promotes the release of IL-6, tumor necrosis factor-α (TNF-α), and other proteins, ultimately leading to destructive inflammation[9-10]. To determine whether BMSCs and exosomes also play a therapeutic role in the treatment of gas explosion-induced blast lung injury through HMGB1 and its downstream pathways, we examined the expression of HMGB1. The results showed that the expression of HMGB1 was significantly increased after the gas explosion (Figure 3H–J), whereas the elevation of HMGB1 was reversed after the intervention of BMSCs and exosomes, and immunofluorescence. The results were consistent with those of western blotting and qRT-PCR (Figure 3K, L, M, O). To further explore the role of HMGB1 in lung injury, changes in the HMGB1-binding receptors RAGE and TLR4 were examined. Compared with the control group, the expression levels of RAGE, p38 mitogen-activated protein kinase (p-p38 MAPK), and p-NF-κB increased, while BMSCs and BMSCs-Exo reversed their increases (Figure 3N, P). In addition, the protein levels of TLR4 and myeloid differentiation factor 88 (Myd88) increased, and those of MSCs and BMSCs-Exos decreased (Figure 3Q, R). These results suggest that HMGB1/NF-κB signaling is involved in the inflammatory response and that BMSCs and BMSCs-Exos can play a protective role in mitigating gas explosion-induced blast lung injury by influencing the onset and progression of inflammation.
To summarize, our study showed that BMSC and BMSC-Exo transplantation improved rat lung vascular permeability after injury due to a gas explosion, promoting endothelial cell repair and attenuating alveolar epithelial injury. BMSCs and BMSCs-Exo also mitigated the progression of gas-explosion-induced lung injury in rats by attenuating the inflammatory response, a process that may be related to the inhibition of HMGB1/NF-κB activation; BMSCs and BMSCs-Exo could achieve the same intervention effect. These findings provide insights that may prove useful for the development of therapeutic approaches for the treatment of explosive lung injury.
doi: 10.3967/bes2024.161
Effect and Mechanism of Action of BMSC-derived Exosomes on Gas Explosion-induced Blast Lung Injury
-
Changfu Hao, Wu Yao, Sanqiao Yao and Wenjie Ren designed the study and applied for approval by the Research Ethics Board. Xiaofei Jin, Hui Fan, and Xuedan Deng provided the experimental support. Xiaoying Li, Meng Deng, and Jing He drafted the manuscript, with important intellectual input from Wu Yao. All the authors approved the final manuscript. All authors had complete access to the study data.
The authors confirm that there are no conflicts of interest.
&These authors contributed equally to this work.
注释:1) Author Contributions: 2) Conflict of Interest: -
Figure 1. Identification of BMSCs and exosomes and modeling of lung injury in gas explosions. (A) An optical microscope (40×) was used to examine the morphology of passage three BMSCs (n = 3; scale bar = 100 µm). (B) Oil Red staining (200×) was used to demonstrate BMSC adipogenic induction (n = 3; scale bar = 100 µm). (C) Alizarin Red staining (200×) was used to demonstrate osteogenic induction of BMSCs (n = 3; scale bar = 100 µm). (D) Flow cytometry detection of CD90, CD29, CD45, and CD11b (n = 3). (E) Transmission electron microscopy was used to examine the morphology of exosomes (TEM) (n = 3; scale bar = 500 nm). (F) Nanoparticle tracking analysis was used to determine the size distribution of exosomes (NTA) (n = 3). (G) Western blot was used to identify exosome markers such as ALIX, TSG101, Calnexin, and CD63 (n = 3). (H) Rat lungs were examined grossly for pathology 72 hours after the explosion (n = 3). (I) Typical H&E staining of rats’ damaged and functional lungs (black arrow: inflammatory cell infiltration, green arrow: thickening of alveolar septum, red arrow: diffuse hemorrhage; n = 3; scale bar = 50 µm). (J) Western blotting was used to quantify IL-1β protein expression in the control and explosion groups (n = 3).
BMSCs, bone marrow-derivedmesenchymal stem cells; TEM, transmission electron microscope; TSG101, tumor susceptibility gene 101; Alix, ALG-2-interacting protein X; IL-1β, Interleukin-1β.
Figure 2. BMSCs and BMSCs-Exo reduce vascular permeability and promote endothelial cell repair while protecting alveolar epithelial cells. (A) Lung W/D ratio in each group 72 hours after the explosion (n = 3). Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (B–C) Western blot results for ZO-1, VE-cadherin, VWF, and CD31 (n = 3). (D) Immunofluorescence results (100×) of ZO-1 and VE-cadherin (n = 3; scale bar = 50 µm). (E–F) Quantitative protein expression of ZO-1, VE-cadherin, VWF, and CD31. Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (G–H) mRNA expression of ZO-1, VE-cadherin, VWF, and CD31 (n = 3). Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (I) mRNA expression of SP-A and SP-C (n = 3). Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (J) Western blot results for SP-A and SP-C (n = 3). (K) Quantitative protein expression of SP-A and SP-C. Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (L) IHC staining for SP-A and SP-C. (200×; n = 3; scale bar = 50 µm).
PBS, phosphate buffer saline; BMSCs, bone marrow-derived mesenchymal stem cells; ZO-1, zonula occludens-1; VE-cadherin, vascular endothelial-cadherin; VWF, von willebrand factor; CD31, platelet endothelial cell adhesion molecule-1; SP, surfactant protein.
Figure 3. BMSCs and BMSCs-Exo effectively alleviate lung injury and inflammation and influence HMGB1/NF-κB activation. (A) Using H&E staining, lung tissue pathological alterations were observed 72 h after the gas explosion (200×; scale bar = 50 µm). (B) An inflammatory score was used to gauge the degree of lung inflammation (n = 10). Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (C) TNF-α, IL-1β, IL-6, and IL-10 mRNA expression in lung tissue (n = 3). Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (D–G) TNF-α, IL-1β, IL-6, and IL-10 protein expression levels in lung tissue were assessed using ELISA (n = 10). Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (H–J) The amount of HMGB1 expressed in the control and explosion groups (n = 3). Data are presented as the mean ± SD, with *P < 0.05 vs. the control group. (K–M) Western blotting and qRT-PCR were used to evaluate changes in HMGB1 expression (n = 3). Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (O) Immunofluorescence detection (200×) of HMGB1 expression level in each group (n = 3). (N) Western blotting was used to evaluate the expression of RAGE, p38 MAPK, p-p38 MAPK, NF-κB, and p-NF-κB proteins (n = 3). (P) Quantification of RAGE, p-p38 MAPK, and p-NF-κB protein expression in lung tissue. Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group. (Q) Western blotting was used to determine the expression of TLR4, Myd88 NF-κB, and p-NF-κB proteins (n = 3). (R) Quantification of TLR4 and Myd88a protein expression in lung tissue. Data are presented as the mean ± SD, with *P < 0.05 vs. the control group, with #P < 0.05 vs. the explosion group.
BMSCs, bone marrowmesenchymal stem cells;IL, interleukin; TNF-α,tumor necrosis factor-α; HMGB1, high mobility group protein B1; RAGE, receptor for advanced glycation end-products; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor kappa-B; TLR4, toll-like receptor 4; Myd88, myeloid differentiation factor 88.
-
[1] Smith JE, Garner J. Pathophysiology of primary blast injury. J R Army Med Corps, 2019; 165, 57−62. doi: 10.1136/jramc-2018-001058 [2] Xu SG, Liu C, Ji HL. Concise review: therapeutic potential of the mesenchymal stem cell derived secretome and extracellular vesicles for radiation-induced lung injury: progress and hypotheses. Stem Cells Transl Med, 2019; 8, 344−54. doi: 10.1002/sctm.18-0038 [3] Liu X, Gao CJ, Wang Y, et al. BMSC-derived exosomes ameliorate LPS-induced acute lung injury by miR-384-5p-controlled alveolar macrophage autophagy. Oxid Med Cell Longev, 2021; 2021, 9973457. doi: 10.1155/2021/9973457 [4] He YT, Chen DM, Yang LL, et al. The therapeutic potential of bone marrow mesenchymal stem cells in premature ovarian failure. Stem Cell Res Ther, 2018; 9, 263. doi: 10.1186/s13287-018-1008-9 [5] Lu GD, Cheng P, Liu T, et al. BMSC-derived exosomal miR-29a promotes angiogenesis and osteogenesis. Front Cell Dev Biol, 2020; 8, 608521. doi: 10.3389/fcell.2020.608521 [6] Nita I, Hostettler K, Tamo L, et al. Hepatocyte growth factor secreted by bone marrow stem cell reduce ER stress and improves repair in alveolar epithelial II cells. Sci Rep, 2017; 7, 41901. doi: 10.1038/srep41901 [7] Alcorn JF, Wright JR. Surfactant protein A inhibits alveolar macrophage cytokine production by CD14-independent pathway. Am J Physiol Lung Cell Mol Physiol, 2004; 286, L129−36. doi: 10.1152/ajplung.00427.2002 [8] Baris SA, Vural C, Yaprak B, et al. The effects of sildenafil on smoke induced lung inflammation in rats. Malays J Pathol, 2016; 38, 39−44. [9] Liang Y, Hou CC, Kong JL, et al. HMGB1 binding to receptor for advanced glycation end products enhances inflammatory responses of human bronchial epithelial cells by activating p38 MAPK and ERK1/2. Mol Cell Biochem, 2015; 405, 63−71. doi: 10.1007/s11010-015-2396-0 [10] He ZW, Qin YH, Wang ZW, et al. HMGB1 acts in synergy with lipopolysaccharide in activating rheumatoid synovial fibroblasts via p38 MAPK and NF-κB signaling pathways. Mediators Inflamm, 2013; 2013, 596716. -




 下载:
下载:



 Quick Links
Quick Links