-
Cold exposure is a pervasive stressor in the polar and subpolar regions, exerting both acute and chronic effects on individuals. This environmental factor is known to induce physiological stress, compromise immune response efficacy, and increase susceptibility to various diseases. Chronic cold exposure, characterized by repetitive non-consecutive exposure to suboptimal temperatures over an extended duration, has the potential to disrupt homeostatic equilibrium, thereby exerting a detrimental influence on overall physiological well-being. The gastrointestinal tract, particularly the intestine, possesses an extensive mucosal layer that serves as the primary interface with the external environment and harbors diverse microbial populations. The intricate nature of this microenvironment makes the intestine highly susceptible to environmental perturbations, ultimately leading to a spectrum of intestinal diseases. A comprehensive investigation into the interplay between chronic cold exposure and the intricate dynamics of the intestinal microenvironment is imperative. This inquiry contributes to our understanding of the physiological consequences of prolonged cold exposure and sheds light on the broader implications of environmental stressors on human health.
Mechanical barriers play crucial roles in maintaining the structural foundation necessary for regulating barrier function. Tight junctions are essential regulators of selective permeability and play an indispensable role in preserving intestinal barrier function. These proteins exhibit remarkable adaptability to complex environments, ensuring dynamic stabilization of the intestinal structure. Notably, changes in their expression and structure occur in response to various intestinal diseases. Upon compromise of the integrity of the intestinal barrier function, which results in an escalation in intestinal permeability, there arises the potential for the ingress of intestinal bacteria and toxins into the bloodstream. This can result in sepsis and, in severe cases, secondary systemic inflammation. Tight junctions play a crucial role in barrier function by forming multiprotein complexes encompassing membrane proteins such as Occludin, Claudins, and Zonula occludens (ZOs). These proteins intricately connect adjacent cells to the actin backbone, and collectively constitute the mechanical barrier of the intestinal epithelium[1].
Chronic inflammation is a hallmark of inflammatory bowel disease (IBD); and the interplay between endoplasmic reticulum (ER) stress and unfolded protein response (UPR) signaling has emerged as a key contributor to the potentiation or prolongation of inflammatory responses. The heightened demand for protein secretion in intestinal tissue necessitates a robust ER to meet these requirements. However, the susceptibility of ER to cellular stress poses a significant burden on its functionality. The accumulation of misfolded proteins in the ER lumen, surpassing a critical threshold, triggers the activation of the UPR signaling pathway to restore cellular homeostasis. Inadequate resolution of ER stress contributes to the pathogenesis of IBD[2]. A growing body of evidence suggests that the UPR may function as a mechanistic driver of proinflammatory processes. Previous studies have consistently emphasized autophagy as a primary pro-survival mechanism for alleviating ER stress.
Consequently, in response to the increased prevalence of intestinal diseases resulting from cold exposure, this study focused on the response of mouse ileal tissues to cold exposure. These findings provide a scientific basis and treatment strategies for the prevention and treatment of intestinal diseases caused by cold stress.
Maintaining temperature equilibrium within the gastrointestinal tract[3] supports the hypothesis that exposure to low temperatures may have detrimental effects on the intestine, thereby increasing susceptibility to intestinal diseases. Observable symptoms such as cold-induced diarrhea or discomfort after consuming cold beverages highlight this vulnerability. To thoroughly evaluate the consequences of prolonged exposure to low temperatures on murine ileum tissue, six male C57bL6 mice (aged five weeks, weighing approximately 20 g) were randomly selected for daily sessions of cold stimulation lasting three hours each, conducted at a temperature of 4 °C for 10 d. After treatment, anesthesia was administered using glutaraldehyde and euthanasia was performed. Collected samples were then appropriately stored at –80 °C for subsequent analysis.
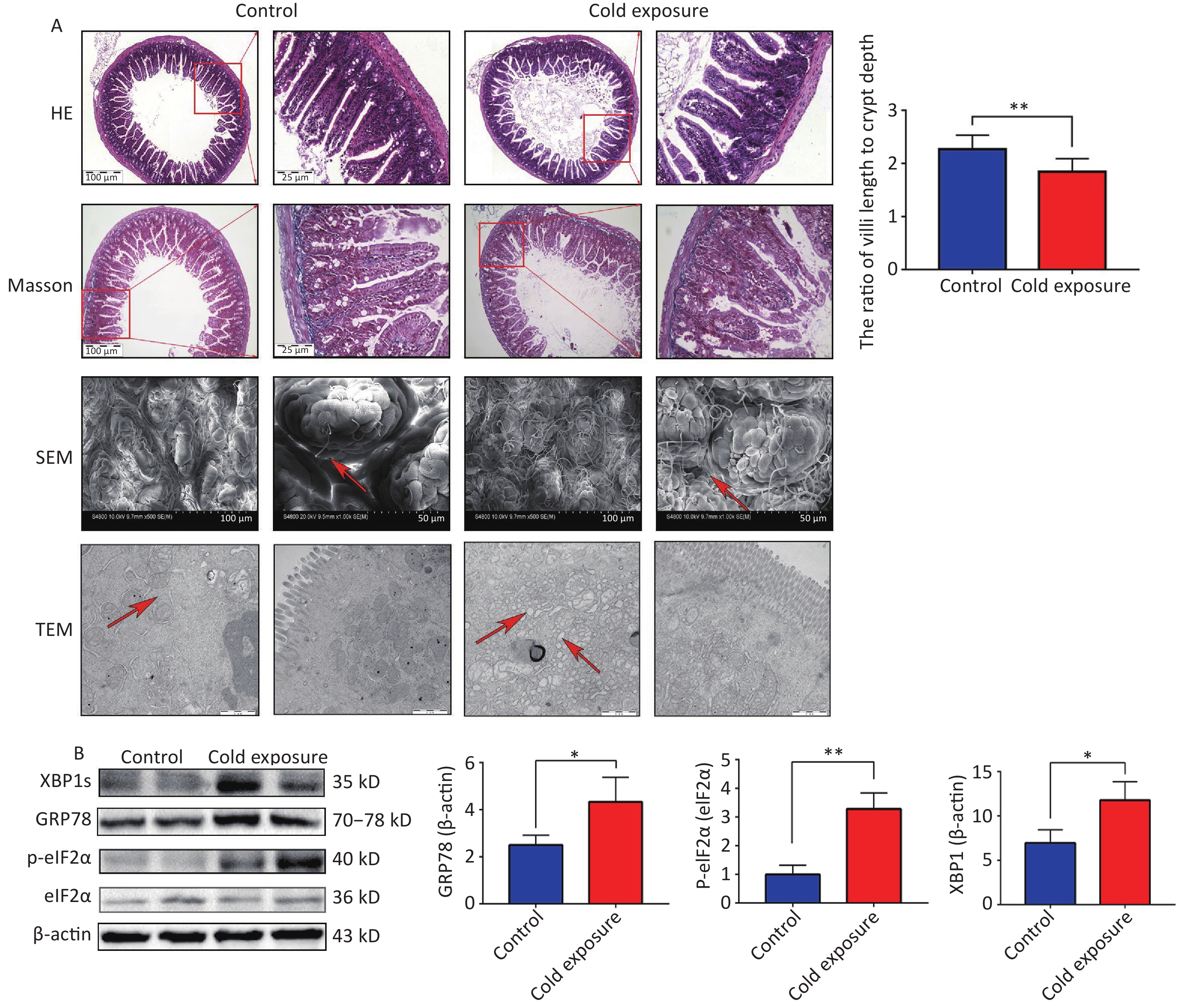
In our study, hematoxylin and eosin (H&E) staining was used to examine the structural consequences of chronic cold exposure on mouse ileal segments. Comparative analysis with a control group revealed a significant decrease in the ratio of villus length to crypt depth within the ileum following chronic cold exposure (Figure 1A). H&E staining revealed thinning of the muscle layer and subtle infiltration of inflammatory cells into the lamina propria due to chronic cold exposure. However, Masson staining did not reveal any significant fibrosis. Analysis using scanning electron microscopy (SEM) revealed distinct alterations to the ileum tissue following cold exposure, including narrowing of the villus gap, rough and uneven surface morphology, and a disorganized villus structure. Transmission electron microscopy (TEM) revealed swelling of the ER in the intestine, which is considered a pivotal indicator of ER stress. To further investigate indicators related to ER stress, Western blotting (WB) was used to assess protein expression. The results demonstrated an upregulation in the expression levels of GRP78, XBP1s, CHOP, and phosphorylated eIF2α in the cold exposure group compared to the control group (Figure 1B). Intestinal tissue, renowned for its highly developed ER structure, plays a crucial role in orchestrating protein synthesis and folding. Exposure to cold temperatures augments the ER workload, activating ER stress in response to environmental changes. The UPR, an adaptive cellular mechanism triggered by ER stress, is responsible for eliminating misfolded proteins. However, persistent ER stress can induce apoptotic cell death and initiate signaling cascades that propagate cellular death. Emerging evidence suggests that 4-phenyl butyric acid (4-PBA) and bovine ursodeoxycholic acid are inhibitors of ER stress and ameliorate intestinal inflammation in models of IBD by alleviating ER stress and apoptosis in intestinal epithelial cells[4].
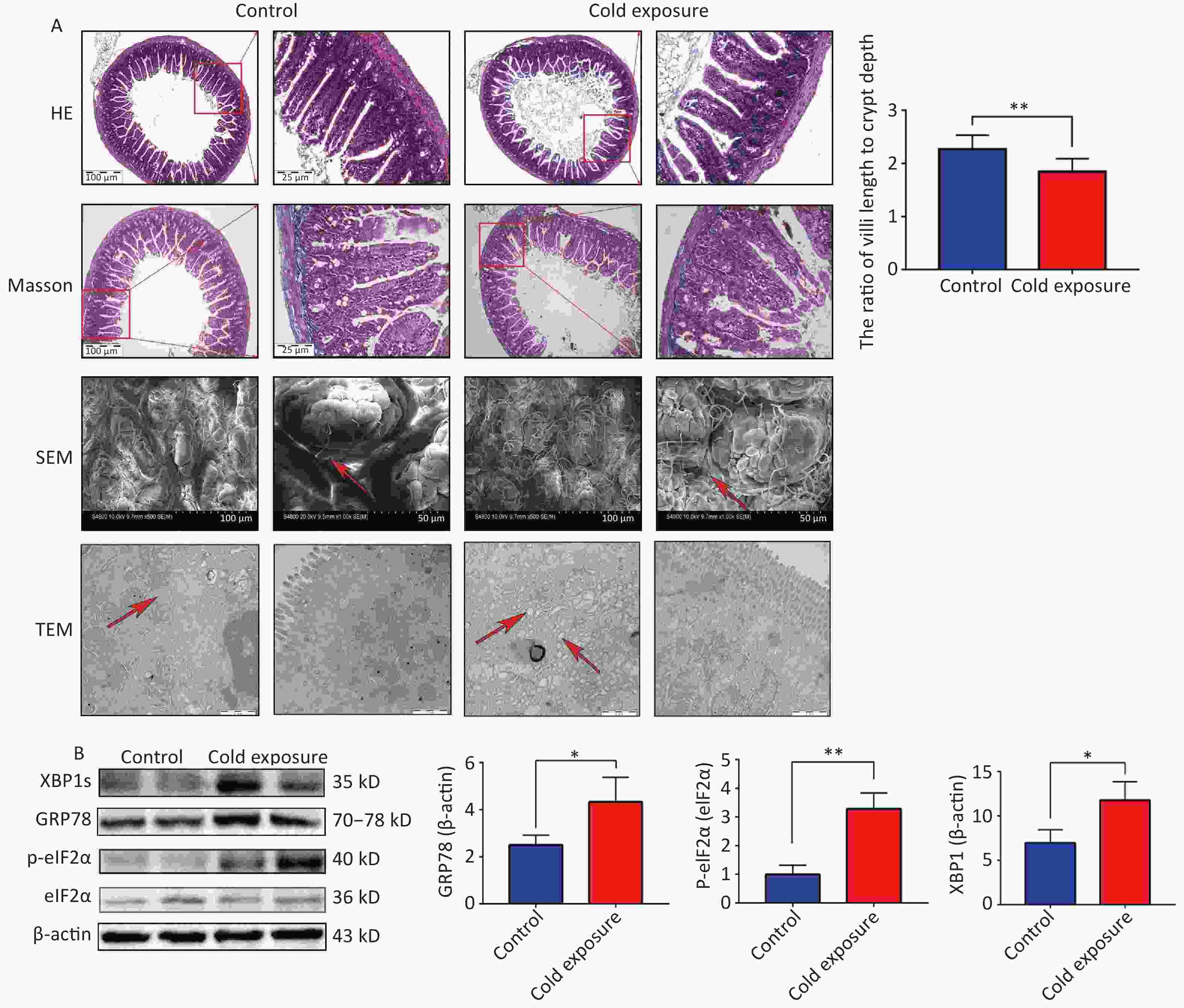
Figure 1. The structural and western blotting (WB) consequences of chronic cold exposure on mouse ileum. (A) Hematoxylin and eosin (H&E) staining, Masson staining (100× and 40×), scanning electronmicroscopy (SEM, scale bars: 50 µm and 100 µm), transmission electron microscopy (TEM, scale bar: 1 µm), and ratio of villi length to crypt depth of ileum tissue (six villi were randomly selected in the visual field of view). (B) Expression of GRP78, XBP1s, p-eIF2α in mouse ileum tissue. *P < 0.05, **P < 0.01.
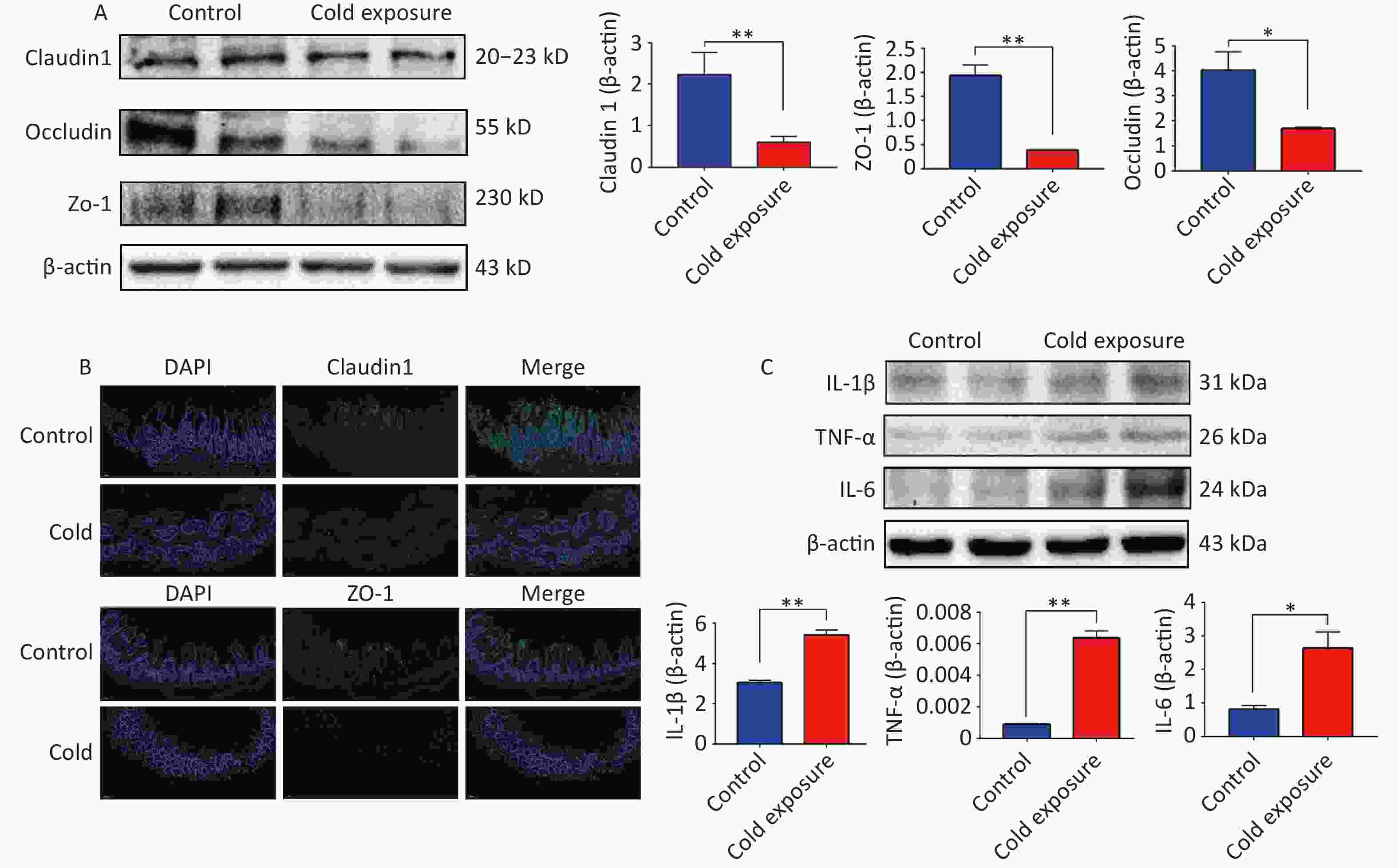
The intestinal epithelial barrier, which is primarily composed of tight junctions, plays a pivotal role in regulating the paracellular flux and epithelial permeability to maintain barrier function. ZO-1 is crucial for tight junction assembly and contributes to cytoskeletal organization. Its interaction with Claudins, Occludin, and junctional adhesion molecules is instrumental in maintaining intestinal barrier integrity[5]. Notably, our observations revealed a downregulation in the expression of tight junction proteins ZO-1, Occludin, and Claudin-1 in the ileal tissues following chronic cold exposure (Supplementary Figure S1A–B, available in www.besjournal.com). This was accompanied by an upregulation of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α (Supplementary Figure S1C), indicating the detrimental impact of chronic cold exposure on the structural integrity and mechanical barrier function of the ileum in mice.
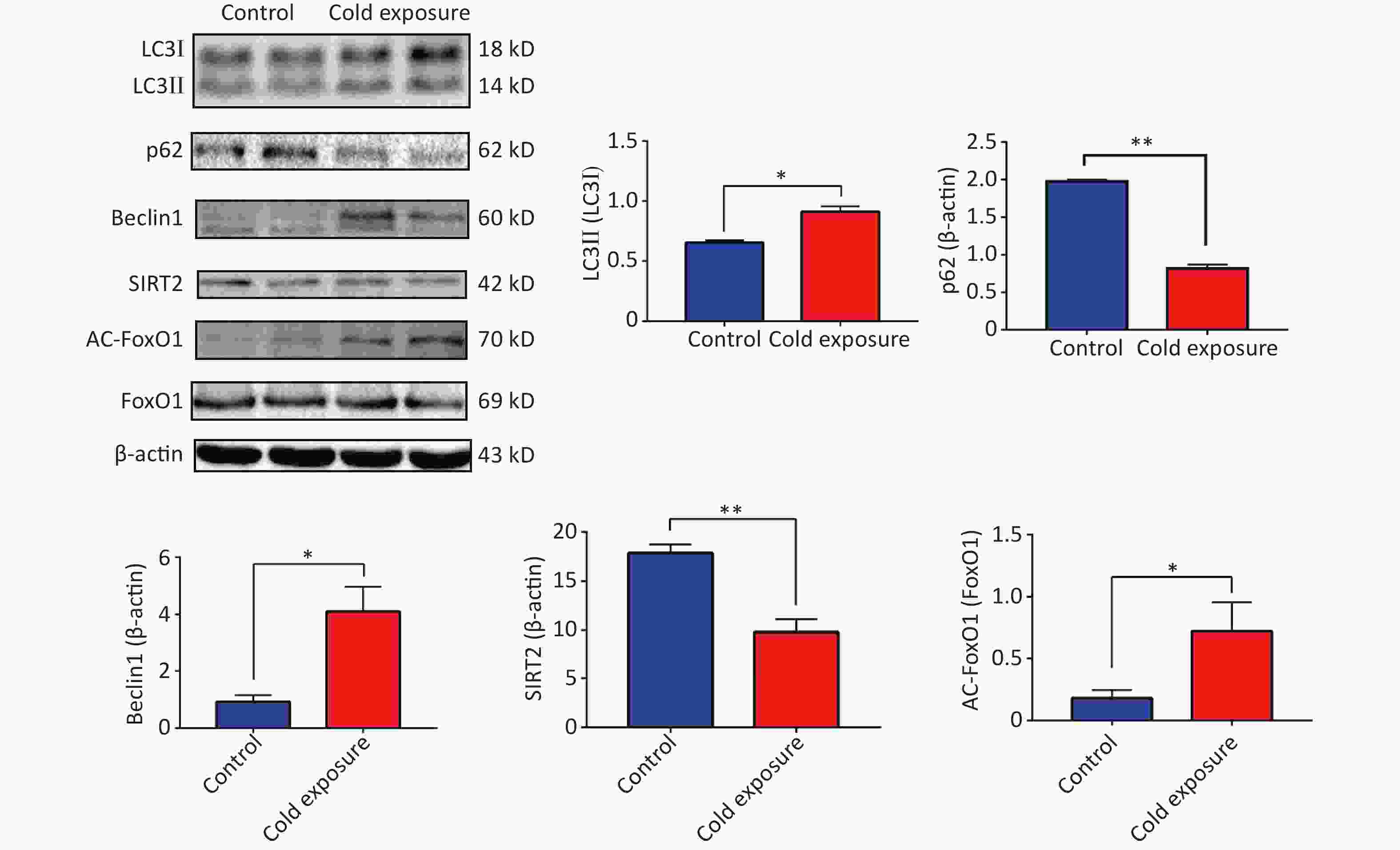
Silencing regulatory factor 2 (SIRT2), a member of the Sirtuin family, influences the acetylation of downstream target genes and participates in various biological processes, including inflammation, autophagy, cell cycle regulation, aging, and stress. By regulating the acetylation of NF-κB p65 subunit, which is closely associated with inflammation, SIRT2 can activate inflammatory NF-κB[6]. Our observations following murine cold exposure indicated downregulation of SIRT2 expression and upregulation of acetylation of its downstream target, FoxO1, in the mouse ileum, suggesting the activation of autophagy. Protein expression levels of Beclin1 increased along with an elevated ratio of LC3II/I and a decrease in p62 levels (Supplementary Figure S2, available in www.besjournal.com). These findings highlight the dynamic interplay between autophagy and ER stress and emphasize their critical roles in both physiological and pathological contexts.
To gain deeper insights into the underlying mechanisms, we aimed to replicate in vitro the conditions of chronic cold exposure by simulating the effects observed in vivo. To achieve this, we co-treated MODE-K mouse small intestinal epithelial cells with the ER stress agonists thapsigargin (Tg) and sh-SIRT2. Our established ER stress model demonstrated a decrease in the expression of tight junction proteins ZO-1, Occludin, and Claudin-1 (Figure 2A–B). Considering the crucial role of autophagy in maintaining intestinal homeostasis, regulating interactions with intestinal microbiota, and contributing to host defense against intestinal pathogens[7], we modulated autophagy using agonists and inhibitors. Our findings revealed that mitigating autophagy impaired ZO-1, a critical indicator of intestinal mechanical barrier integrity (Figure 2C). Moreover, under ER stress conditions, SIRT2 knockdown significantly increased FoxO1 acetylation and enhanced autophagy (Figure 3), highlighting the intricate relationship between SIRT2, autophagy, and intestinal epithelial barrier maintenance. Autophagy can regulate the expression of tight junctions to maintain intestinal epithelial permeability[8], indicating a protective role of autophagy in intestinal epithelial barrier function[9]. It is worth noting that while autophagy disrupts the integrity of the intestinal barrier in severe burns[10], our study, in conjunction with previous research, suggests that this discrepancy may be influenced by the overall physiological stress state and extent of autophagic activity. In situations where systemic compromise is profound and irreparable, autophagy facilitates cell death through the phagocytosis of damaged cells.
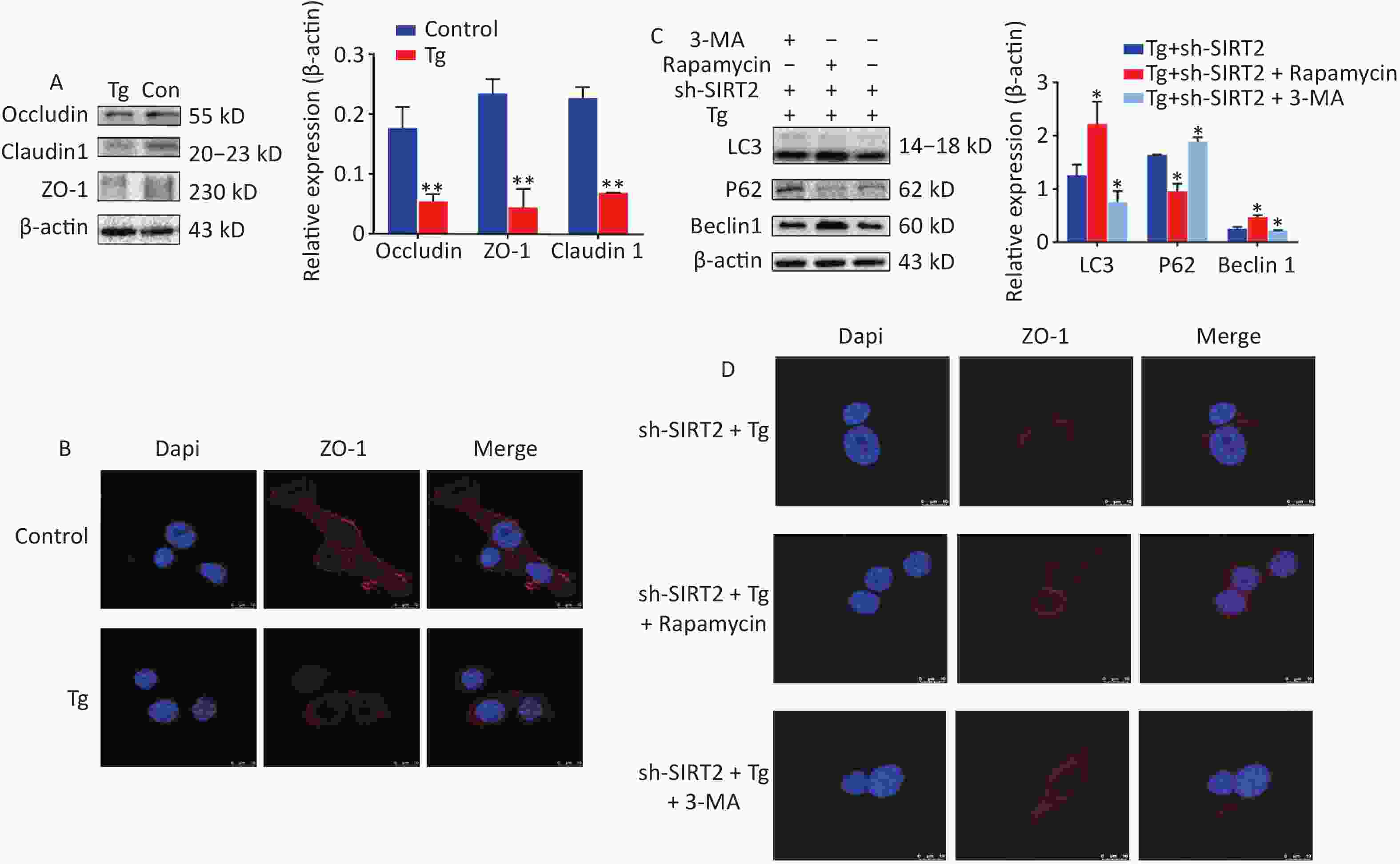
Figure 2. The expression and Immunofluorescence of proteins under endoplasmic reticulum (ER) stress model. (A) Expression of ZO-1, Occludin and Claudin-1 in MODE-K under Tg and sh-SIRT2 treatment. *P < 0.05, **P < 0.01. (B) Immunofluorescence of ZO-1. (C) Expression of LC3, Beclin 1, and p-eIF2α wafterith Rapamycin and 3-MA treatment. (D) Immunofluorescence of ZO-1 after Rapamycin and 3-MA exposure.
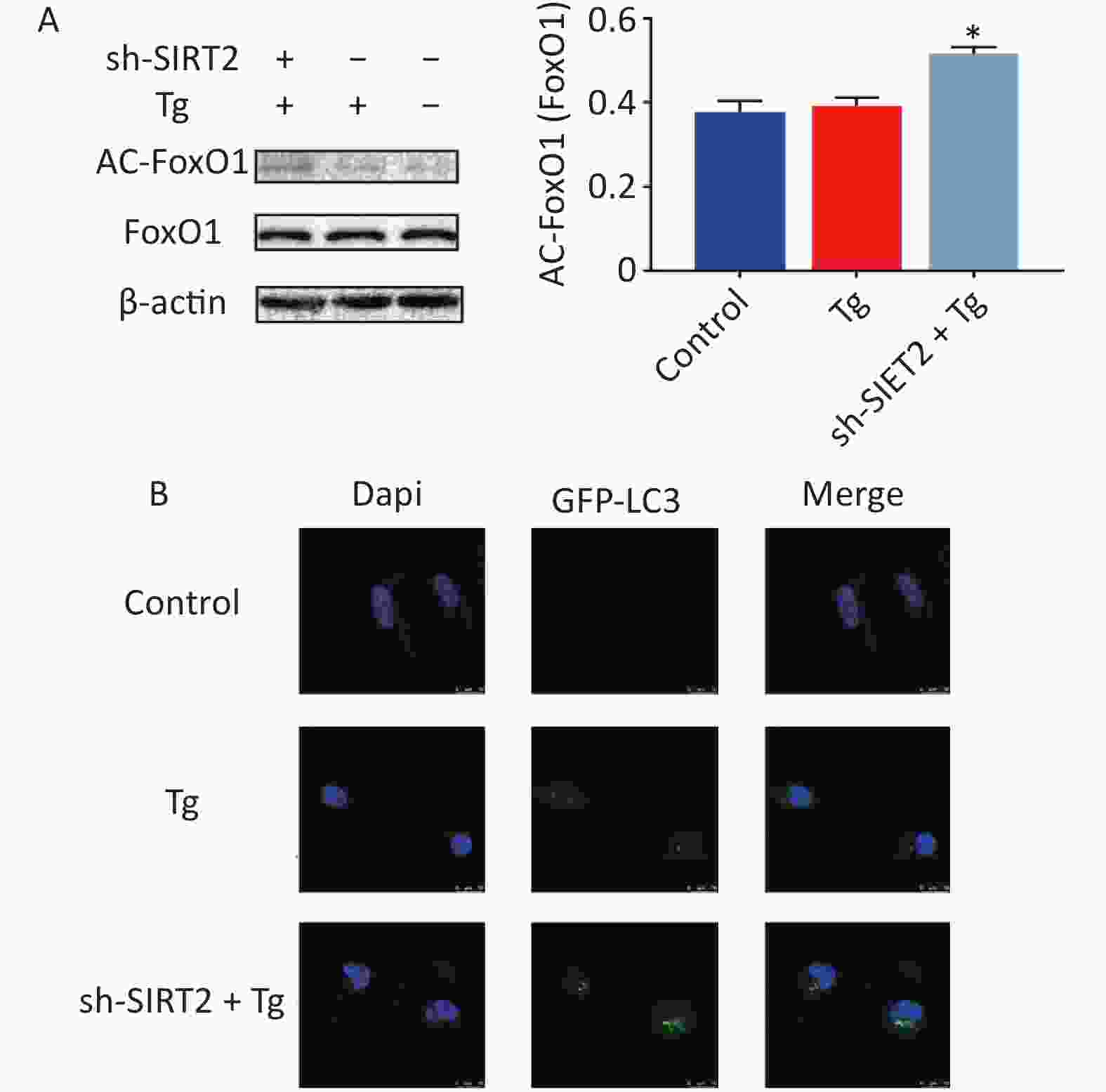
Figure 3. The expression of proteins under endoplasmic reticulum (ER) stress model. (A) Expression of AC-FoxO1 after Tg and sh-SIRT2 treatment. (B) GFP-LC3 flux after Tg and sh-SIRT2 treatment (scale bar: 10 µm). *P < 0.05, **P < 0.01.
Extended durations of chronic cold stimuli induce compensatory and adaptive responses in organisms, differentiating them from acute cold exposure scenarios. These responses are coordinated to maintain homeostasis and effectively delay the onset of injury. In our study, we observed that SIRT2 downregulation occurred as an adaptive response triggered by chronic cold exposure, subsequently resulting in the upregulation of autophagy. This coordinated response enhances the integrity of the intestinal mechanical barrier. In summary, our findings indicate that chronic cold exposure induces ER stress and compromises the integrity of tight junctions in the ileum. Importantly, the observed downregulation of SIRT2 expression during stress emerges as a pivotal modulator, enhancing autophagy and mitigating damage to tight junctions. This adaptive response sheds light on the intricate mechanisms underlying an organism’s ability to cope with prolonged cold exposure. It also highlights the dynamic interplay between SIRT2, autophagy, and maintenance of intestinal barrier function. This study had certain limitations. We found that SIRT2 expression was adaptively regulated during the selected chronic cold exposure treatment period in this study and contributed to the delay of injury. We plan to add time points and set cold exposure for 10 d, 20 d and 30 d to investigate the dynamic interplay between SIRT2, autophagy, and maintenance of intestinal barrier function.
No potential conflicts of interest were disclosed.
Jing Xu completed the experimental design and paper writing. Jingru Guo completed the experimental design and paper writing. Leichong Chen, Huijie Hu, and Junshu Nie, Jianbin Yuan, Li Ma, Jingjing Lu completed the intestinal function test. Hong Ji completed the experimental design. Bin Xu completed the experimental design and financial support.
doi: 10.3967/bes2024.120
-
&These authors contributed equally to this work.
注释: -
Figure 1. The structural and western blotting (WB) consequences of chronic cold exposure on mouse ileum. (A) Hematoxylin and eosin (H&E) staining, Masson staining (100× and 40×), scanning electronmicroscopy (SEM, scale bars: 50 µm and 100 µm), transmission electron microscopy (TEM, scale bar: 1 µm), and ratio of villi length to crypt depth of ileum tissue (six villi were randomly selected in the visual field of view). (B) Expression of GRP78, XBP1s, p-eIF2α in mouse ileum tissue. *P < 0.05, **P < 0.01.
Figure 2. The expression and Immunofluorescence of proteins under endoplasmic reticulum (ER) stress model. (A) Expression of ZO-1, Occludin and Claudin-1 in MODE-K under Tg and sh-SIRT2 treatment. *P < 0.05, **P < 0.01. (B) Immunofluorescence of ZO-1. (C) Expression of LC3, Beclin 1, and p-eIF2α wafterith Rapamycin and 3-MA treatment. (D) Immunofluorescence of ZO-1 after Rapamycin and 3-MA exposure.
-
[1] Kim S, Kim GH. Roles of claudin-2, ZO-1 and occludin in leaky HK-2 cells. PLoS One, 2017; 12, e0189221. doi: 10.1371/journal.pone.0189221 [2] Eugene SP, Reddy VS, Trinath J. Endoplasmic reticulum stress and intestinal inflammation: a perilous union. Front Immunol, 2020; 11, 543022. doi: 10.3389/fimmu.2020.543022 [3] Roxane B, Chandrou KO, Alexandre CP, et al. Gastrointestinal thermal homogeneity and effect of cold water ingestion. J Therm Biol, 2018; 78, 204−8. doi: 10.1016/j.jtherbio.2018.10.002 [4] Kim S, Lee S, Lee H, et al. A colon-targeted prodrug, 4-phenylbutyric acid-glutamic acid conjugate, ameliorates 2, 4-dinitrobenzenesulfonic acid-induced colitis in rats. Pharmaceutics, 2020; 12, 843. doi: 10.3390/pharmaceutics12090843 [5] Bhat AA, Uppada S, Achkar IW, et al. Tight junction proteins and signaling pathways in cancer and inflammation: a functional crosstalk. Front Physiol, 2019; 9, 1942. doi: 10.3389/fphys.2018.01942 [6] Wang XF, Buechler NL, Martin A, et al. Sirtuin-2 regulates sepsis inflammation in ob/ob mice. PLoS One, 2016; 11, e0160431. doi: 10.1371/journal.pone.0160431 [7] Mizushima N. A brief history of autophagy from cell biology to physiology and disease. Nat Cell Biol, 2018; 20, 521−7. doi: 10.1038/s41556-018-0092-5 [8] Zhang C, Yan JK, Xiao YT, et al. Inhibition of autophagic degradation process contributes to claudin-2 expression increase and epithelial tight junction dysfunction in TNF-α treated cell monolayers. Int J Mol Sci, 2017; 18, 157. doi: 10.3390/ijms18010157 [9] Nighot PK, Hu CAA, Ma TY. Autophagy enhances intestinal epithelial tight junction barrier function by targeting claudin-2 protein degradation. J Biol Chem, 2015; 290, 7234−46. doi: 10.1074/jbc.M114.597492 [10] Huang YL, Wang Y, Feng YH, et al. Role of endoplasmic reticulum stress-autophagy axis in severe burn-induced intestinal tight junction barrier dysfunction in mice. Front Physiol, 2019; 10, 606. doi: 10.3389/fphys.2019.00606 -
 23360+Supplementary Materials.pdf
23360+Supplementary Materials.pdf

-




 下载:
下载:





 Quick Links
Quick Links