-
Indoor air conditions can sometimes be detrimental to human health. For example, sick building syndrome is a well-known illness induced by certain indoor air conditions[1-3]. Many chemical substances cause non-specific symptoms such as general fatigue, headaches, eye, nose, and throat irritation, dizziness, and nausea. Although various biomedical investigations have been reported[4-6], the best way to prevent the occurrence of this syndrome is to avoid exposure to chemicals that induce this illness in sensitive individuals[1-3, 6-7]. Additionally, some respiratory allergic syndromes such as hypersensitive pneumonitis and bronchial asthma are also known to be caused by indoor air pollutants[9-11]. Furthermore, differences in temperature between individual rooms can cause hypertension, sleep disturbances, and life-style related diseases[12-14].
Many housing manufacturers have been working towards the establishment of living conditions that prevent these aforementioned illnesses, such as ensuring the air tightness, stable temperatures throughout the entire home, and high thermal insulation. These measures might help to promote health in addition to avoiding a decline in general health.
We previously developed a negatively charged particle-dominant indoor air conditions (NCPDIAC) device[15]. The device utilized generated particles, approximately 20 nm in diameter, which were created by initially painting fine charcoal powder onto the walls and ceiling of a room. The charcoal coating, designated Health Coat, was produced by Artech Kohbou Co. Ltd., Higashisonogi, Nagasaki, Japan. Additionally, a forced negatively charged particle-dominant indoor air condition was created by applying an electric voltage (72 V) between the backside of the walls and the ground, as shown in Supplemental Figure 1A (available in www.besjournal.com), and as previously reported[15]. For the initial trial to examine the biological effects of NCPDIAC, control and experimental (NCPDIAC) rooms were built, and it was found that indoor factors such as temperature, humidity, and concentration of chemicals including formaldehyde and other volatile organic compounds did not differ between control and NCPDIAC rooms[15]. In experimental trials consisting of short-term (2.5 h) stays, there were only slight differences in outcomes between stays in control and NCPDIAC rooms; however, a significant increase in interleukin (IL)-2 was detected in the NCPDIAC group, as shown in Supplemental Figure 1B[15]. Additionally, a formula formed by multiple regression analysis indicated that there are clear differences between individuals who stayed in control and experimental NCPDIAC rooms, as previously reported[15]. Thus, to examine this system in ordinary homes, an extended stay investigation was performed. A study comprising nightly stays for two weeks (mid-term) was performed as shown in Supplemental Figure 1C[16]. Participants in the mid-term study comprised 15 males, and at completion of the study showed an increase in natural killer (NK) cell activity (Supplemental Figure 1C), as previously reported[16]. It was expected that although short-term stays with NCPDIAC result in slight and short-term increases in IL-2, repeated and recurrent accumulation of IL-2 levels in the night-time mid-term group would result in NK cell activation since IL-2 is the most effective activating cytokine for these cells (Supplemental Figure 1D)[15-16]. In these experiments, the difference in the number of negatively charged particles in the rooms was approximately 500 particles per 1 cc of air volume[15-16].
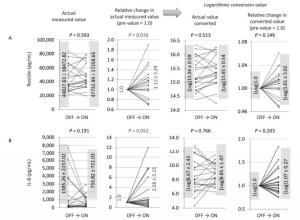
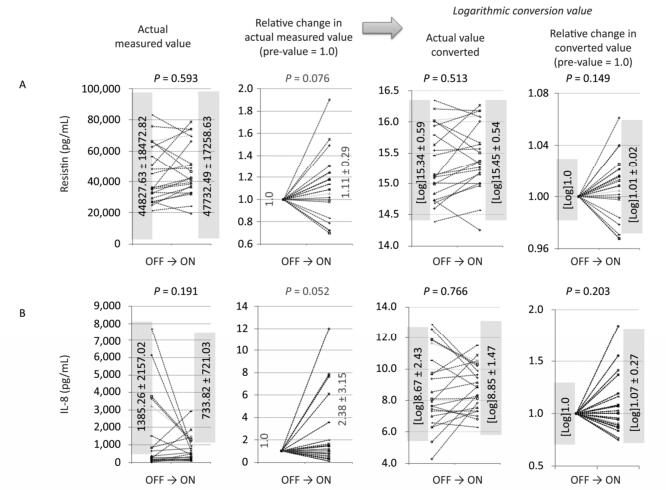
Figure 1. Changes in actual measured values, relative changes in actual measured values (pre-value as 1.0), logarithmic converted values of actual values, and relative changes in values of ON trials observed for resistin (A) and IL-8 (B). For both cytokines, the relative changes in actual measured values tended to increase (0.5 < P < 1.0), although other analyses revealed no statistical significance or tendencies.
Additional experimental approaches were employed as shown in Supplemental Figure 1E and as previously reported[17]. In these studies, negatively charged air particles were continuously forced into and out of a cell culture incubator. Peripheral blood mononuclear cells (PBMCs) were collected from healthy volunteers and placed in a standard incubator or a negatively charged particle-dominant air condition (NC) incubator[17]. The difference in negatively charged particles was approximately 3, 000 particles per 1 cc of air volume in the incubators[17]. Although the number of particles differed from typical room conditions, the findings showed T cell activation (increased expression of CD25 in CD4+ T cells), increased interferon (IFN)-γ production in PBMC cultures, and enhanced NK cell activity. Additionally, the immune index, calculated by the formula log (NK activity × IFN-γ concentration in culture supernatants × IL-2 production in supernatants)/IL-10 concentration, increased significantly under NC incubator conditions[17]. These findings indicated that NCPDIAC also induces immune-stimulation[17]. Since marked adverse effects were not experienced or expected in short-term (2.5 h)[15], mid-term (two weeks, nightly stays)[16], and in vitro experimental trials[17], the present study proceeded to observe the biological effects of NCPDIAC under actual living conditions for a period of three months[18]. As previously reported[18] and shown in Supplemental Figure 2A-B (available in www.besjournal.com), seven volunteers were subjected to NCPDIAC in the bedroom or living room of their home; before study initiation, biological parameters were measured[18]. Thereafter, subjects switched the NCPDIAC device ON or OFF every three months (long-term) and then provided blood and urine samples for biological monitoring. As a result, the relative NK cell activity was found to increase during the three-month ON trials and decrease during the three-month OFF trials. NK cell activity was measured with NK cell to target cell ratios of 10:1 or 20:1, as shown in Supplemental Figure 2C and as previously reported[18].
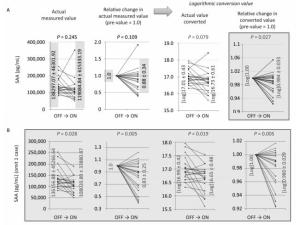
Figure 2. Changes in actual measured values, relative changes in actual measured values (pre-value as 1.0), logarithmic converted values of actual values, and relative changes in values of ON trials observed for SAA (A), and SAA with one case omitted, which showed an exceptionally marked increase (B). In all 16 ON trials, logarithmic converted actual values showed a tendency to decrease, and logarithmic converted relative changes showed a significant decrease in SAA levels (A). Additionally, with the omission of one case that showed an exceptionally marked increase in SAA, all other cases revealed a significant decrease in SAA (B).
Some clients who had utilized Health Coat produced by Artech Kohbou Co. Ltd., without an applied electric voltage, reported improvements in their diabetes mellitus conditions. Since this device includes an underground adapter, there is a certain electric voltage between the living area of the home and the underground that resembles the forced electric voltage described above, although this voltage is unstable. To evaluate the feelings experienced by volunteers, adipokines and cytokines related to oxidative stress (Supplemental Figure 2D) were measured in serum derived from volunteers participating in the aforementioned long-term experimental study.
-
As previously reported[18], of the seven volunteers that had utilized the NCPDIAC device, five had the device in their bedroom and two had it in their living room. Details of the subjects were included in our previous report[18]. During experiment periods, there were no reports of viral or bacterial infections in volunteers.
All seven healthy volunteers were Japanese individuals living in Japan. The average age of the volunteers was 54.86 ± 9.15 years, including five males and two females. All volunteers built or reconstructed their residential home before being recruited into this project and agreed to utilize the NCPDIAC device for these experiments. The NCPDIAC devices were provided by Artech Kohbou Ltd. and Yamada SXL Home Co. Ltd. The experiment was approved by the Ethical Committee of Kawasaki Medical School and associated hospital, Kurashiki, Japan, and written informed consent was obtained from all volunteers prior to the commencement of experiments.
Samples were taken every three months as shown in Supplemental Figure 2B. As indicated in our previous report[18], volunteers switched the NCPDIAC device ON or OFF every three months immediately after blood and urine samples were taken for biological monitoring. All seven volunteers were absent from their NCPDIAC-equipped room during the day, as they worked elsewhere, and were only subjected to NCPDIAC in the evenings. After pre-sampling of blood and urine, all volunteers switched the NCPDIAC device ON and then switched it either OFF or ON every three months. A total of 16 trials, comprising 'OFF to three months ON' (ON trials) periods, were safely conducted, in addition to 13 trials comprising 'ON to three months OFF' (OFF trials) periods. The longest trials consisted of two volunteers who completed three ON trials and three OFF trials over a period of 1 year and 9 months[18]. The shortest trial consisted of one volunteer who completed only one ON trial and one OFF trial before the volunteer was subsequently transferred to another location by their employer[18].
-
As we reported previously[18], the NICPDIAC apparatus was placed in each room housing of the volunteers. It had an approximate size of 30(H) cm × 20(W) cm × 4(D) cm. In addition, the blood samples used in this study were remaining serum samples from our previous report[18], in which sera were taken to examine blood chemistry including liver (AST, ALT, and γGTP) and kidney (BUN and creatinine) functions, blood sugar, HbA1c, and peripheral blood counts including differential white blood cell counts, as well as 26 different cytokines measured by the Luminex 26 Cytokine Plex Kit Human Cytokine/Chemokine Panel 1 (MPXHCYTO60KOMX26, Merck, Milliopore, Billerica, MA). Although in a previous study[18], other biological markers such as NK cell activity and immunological markers (immunoglobulins and allergen-specific IgEs) were measured, the results have already been reported[18]. In addition, except NK cell activity, no parameters changed according to the ON and OFF trials, including blood sample parameters and 8-OhdG in urine samples.
-
Our previous report[18] detailed the difference in particle concentration between ON and OFF NCPDIAC. As reported[15-16], positively and negatively charged particles in six representative NCPDIAC-equipped rooms were measured using as Ion Counter (EM-1000, Eco Holistic Inc., Suita, Japan). As with previous reports concerning short- and mid-term experiments, the difference in positively and negatively charged particles was approximately 500 particles per cc of air volume, even when the device was utilized in occupied homes[18].
-
Adipokines and cytokines related to oxidative stress in sera from 16 ON and 13 OFF trials were measured using the Human Adipokine Magnetic 14-Plex Panel (Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA, USA) with Luminex instruments (Bio-rad, Hercules, CA, USA). The 14 cytokines examined were IL-1β, IL-10, IL-6, monocyte chemotactic protein (MCP)-1, leptin, serum amyloid A (SAA), hepatocyte growth factor (HGF), insulin, lipocalin-2, tumor necrosis factor (TNF)-α, B cell activating factor (BAFF: belonging to the tumor necrosis factor family), resistin, plasminogen activator inhibitor (PAI)-1, and IL-8.
-
A comparison of cytokine concentrations [actual data, relative actual data (pre-value as 1.0), logarithmic value, and the ratio of logarithmic values (pre-value as 1.0)] before and after the ON and OFF trials was performed using the Student's t-test (paired sample, double-sided).
For each analysis, a P value less than 0.05 was considered significant and a value between 0.05 and 0.1 was considered indicative of tendency. All statistical analyses were performed using SPSS ver. 22 (IBM Japan, Tokyo, Japan).
-
There were no significant changes or tendencies regarding any of the 14 adipokines and cytokines related to oxidative stress in individuals during the OFF trials.
-
The initial premise of this study was based on the fact that some clients who had utilized Health Coat without an applied electric voltage reported improvements in their diabetes mellitus conditions (as mentioned). Although blood sugars and HbA1c values were monitored, there was no significant increase or decrease according to ON or OFF cycles. Thus, we planned to measure adipocytokines as sub-clinical changes representing metabolic situations in volunteers.
-
For the ON trials, levels of one of the adipokines, resistin (Figure 1A), and one of the cytokines, IL-8 (Figure 1B), tended to decrease when data were analyzed as relative actual changes. Given the variations in the actual values in individual volunteers and individual ON trials, relative changes as pre-values set to 1.0 were analyzed. Additionally, since some cytokines might reflect meaningful changes in logarithmic values, the log of the actual values as well as the log of relative changes were also examined. With resistin and IL-8, only relative changes in actual values tended to increase (0.5 < P < 1.0), for both cytokines, in the ON trials. However, three of the other evaluations (actual value, log of actual, and log of relative) did not show significance or tendencies.
-
Altered SAA levels were only noted for the log of the relative changes, which significantly decreased during the ON trial; the log of the actual values tended to decrease, although unconverted actual values and their relative changes did not show any significance or tendency, as shown in Figure 2A. When examining the actual measured values and associated relative changes, one exceptional case showed a marked increase in SAA. Therefore, the data for this case were excluded from statistical analyses. As a result, all four types of analyses revealed statistical significance, and the ON trials resulted in decreased SAA. However, in the one exceptional case referred to previously, there was no specific information about the volunteer. Thus, we excluded this case, which was positioned as the reference level to the end.
-
NCPDIAC resulted in increased NK cell activity during the three-month ON trials and decreased activity during the OFF trials, as previously reported[16, 18]. These results indicated that NCPDIAC might promote human health, and in particular, prevent cancers and reduce symptoms caused by viral infections. Although extended long-term trials, comprising a number of years, should be conducted, a three-month period representing normal lives might be sufficient to demonstrate the benefits of the NCPDIAC device in terms of health promotion and improvement[18].
The NCPDIAC device consists of a coating of fine charcoal powder on the walls and ceiling of a room in addition to the application of an electric voltage (72 V) between the backside of the walls and the ground, as shown in Supplement Figure 1A[15]. Instead of a forced electric voltage, the initial apparatus utilizes an underground adapter that induces electric differences between rooms and the underground, and these differences were unstable and varied between individual areas. For example, neighborhood homes surrounding the elevated railroad tracks of bullet trains do not show any significant predominance of negatively charged particles within rooms. Thus, the main role of the charcoal powder in this device might be to act as a deodorizer and dehumidifier. However, some clients who utilized a charcoal coating with an underground adapter in the absence of a forced electric voltage reported improvements in their diabetes mellitus conditions. This was evident following our publication[18]. Although these observations relate to the feelings experienced by certain clients, an additional assay was performed. However, it should be noted that HbA1c had previously been monitored and did not show significant change during three-month ON and OFF trials[18]. However, since living conditions generally comprise many years, slight changes in adipokines and cytokines related to oxidative stress might be induced by three-month stays, and could possibly modify the diabetes background of individual healthy volunteers.
As a result, statistical significance was only observed for the log of relative changes in SAA (Figure 2A). Although we presented the analyzed results of resistin and IL-8, the tendencies were only revealed by examining the relative changes in the actual values for both molecules (Figure 1A-B). We should conclude that there were no significant alterations in resistin or IL-8 as a result of three-month stays with NCPDIAC. Resistin is also known as an adipose tissue-specific secretory factor, and a positive correlation between resistin serum levels and obesity status has been demonstrated[19-21]. Resistin is secreted from adipocytes and enhances resistance to insulin, thus causing a deterioration in the diabetes condition as well as arteriosclerosis, inducing upregulation of low-density lipoprotein-cholesterol[19-21]. Thus, although resistin levels tended to increase during the ON trials with NCPDIAC, whether this affects health status could not be concluded. Additionally, the tendency of IL-8 levels to increase was only observed when relative changes in actual values were examined. However, it should be concluded that overall changes in IL-8 levels were not significant. Although IL-8 is one of the most important cytokines required for the chemotaxis of neutrophils and phagocytosis of inflammatory cells such as macrophages and neutrophils[22-23], levels of IL-8 are known to increase with oxidative stress[24-27]. The increase in IL-8 levels might induce further elevation of oxidative stress mediators and result in a positive correlation with obesity. As with resistin, it could not be concluded that the changes in IL-8 levels during the NCPDIAC ON trials affect health status.
Finally, we turn our attention to the results concerning SAA. The results showed significant decreases in SAA when the log of the relative changes were analyzed. Additionally, with the omission of one exceptional case, all cases showed significant decreases in SAA during the ON trials with NCPDIAC, although omission of this special case was not based on volunteer information but on the exceptional distribution of actual and relative values. SAA is known to associate with serum high density lipoprotein (HDL)-cholesterol[28-30]. Although SAA is not associated with the metabolism of HDL-cholesterol, the decrease in SAA might induce a reduction in HDL-cholesterol, thereby resulting in exacerbation of atherosclerosis[28-30]. In contrast, SAA plays a role as an acute phase protein during inflammation, similar to C-reactive protein (CRP)[31-33]. As with CRP, SAA is produced and secreted from hepatocytes and is associated with acute inflammation. As biomarkers of acute inflammation are induced by various acute phase cytokines such as IL-6, TNF-α, and IL-1, these proteins play a role in transporting cholesterol from the liver to the bile[28-30]. Elevated high-sensitive CRP is utilized as a biomarker for assessing increased risk of diabetes, hypertension, and cardiovascular disease[34-37]. High-sensitive CRP levels might mediate the deterioration of cardiovascular events. If this theory is applicable to serum levels of SAA, slight changes in SAA levels, which tended to decrease during the ON trials with NCPDIAC, might indicate a decreased risk of atherosclerosis and cardiovascular events[34-37]. The use of the NCPDIAC device could therefore promote the prevention of these life-endangering events. Further investigations utilizing long-term stays with NCPDIAC and comprising a number of years might support this speculation.
The limitations of this study are as follows: (1) The sample size was low; obtaining a large number of samples was difficult, because setting in rooms of house in which all volunteers were actually purchased and living in there. (2) Even with the small sample size, NK cell activity was significantly increased and decreased in ON and OFF trials, respectively, as previously reported[38]. However, changes in SAA, which was the focus of this study, were significant when one case was omitted. Thus, it is necessary to observe these volunteers for an extended period of time (more than 5 to 10 years) in a permanently ON situation. Thereafter, the occurrence of cardiovascular events should be monitored. In other words, it would be better to recruit other volunteers who agree to be monitored over a longer period of time (again, 5 to 10 years) for blood pressure, lipid analyses and blood sugar, HbA1c, and concentrations of adipocytokines to confirm the preventive effects of NCPDIAC conditions. (3) Although this was a self-controlled study, we considered that NK cell activity (as reported before[38]) and SAA levels could not be altered. (4) Other situations such as smoking, daytime work intensity, and intake of antioxidants, which alter conditions of anti-oxidative stresses were not taken into account. Although NCODIAC is considered to promote health (especially anti-tumor immunity), other adverse life styles such as smoking and mental stresses might counteract the effect of this apparatus. Thus, the results obtained in this study were based on the concept that other adverse conditions might exist for the subjects. However, as NK activity was clearly changed despite these conditions, the observation of adipocytokines and cytokines related to oxidative stress was considered valid. In the future, if a larger number of subjects can be recruited, these conditions related lifestyle might be considered and analyzed in greater detail.
Taken together, there were no significant findings regarding changes in adipokines and cytokines related to oxidative stress during three-month stays with NCPDIAC in this study, although changes in SAA levels should be noted. SAA levels tended to decrease during ON trials with NCODIAC, and this might suggest a role in preventing the progression of arterio-atherosclerosis. Further cohort studies should assist in examining this possibility. In addition to our previous findings indicating that NCODIAC enhances NK cell activity, the NCODIAC device might be useful for improving health conditions in normal individuals.
-
The authors express special appreciation to Messrs. Kazunori Fujimoto and Kazuhisa Ami of Yamada SXL Home Co. Ltd., Maebashi, Gunma, Japan and Messrs. Akira Inui and Takashi Shirahama of Artech Kohbou, Co. Ltd., Higashisonogi, Nagasaki, Japan, for their assistance in establishing the NCPDIAC device in the homes of healthy volunteers. This study was supported by a Kawasaki Medical School Research Grant (28B051).
-
The Department of Hygiene, Kawasaki Medical School, Kurashiki, Okayama, Japan, to which all authors belong, received research funding from Yamada SXL Home Co. Ltd. in the years 2015 and 2016. A portion of the expense incurred for the installation of the NCPDIAC device in individual volunteer homes was provided by Artech Kohbou, Co. Ltd., Higashisonogi, Nagasaki, Japan.
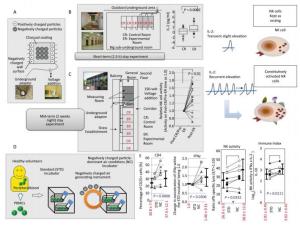
Figure Supplemental Figure 1. Summary of construction, short-term (2.5 h)[15] and mid-term (two weeks, nightly stays) experiments[16] with negatively charged particle-dominant indoor air conditions (NCPDIAC) and in vitro experimental approaches[17]. (A) The construction of NCPDIAC was performed by initially painting fine charcoal powder onto the walls and ceiling of a room[15]. The charcoal coating designated as Health Coat was produced by Artech Kohbou Co. Ltd. The NCPDIAC device was then completed by applying an electric voltage (72 V) between the backside of the walls and the ground[15]. This device generates a slight negative charge at the surface of the wall, which facilitates the adsorption of positively charged particles[15]. Thus, the presence of negatively charged particles predominates and is continuous (particles approximately 20 nm in diameter, and 500 particles per 1 cc of air volume)[15]. (B) The short-term (2.5 h) stay experiments comprising 60 volunteers, with each occupying a control or experimental (NCPDIAC) room, showed slight but significant increases in IL-2 after a 2.5 h stay with NCPDIAC[16]. (C) The mid-term (two weeks, nightly stays) experiments comprising 15 volunteers showed enhanced natural killer (NK) cell activity[16]. Volunteers initially occupied control rooms and thereafter moved to NCPDIAC rooms without being informed of the type of room they were occupying[16]. Differences in various biological markers were then analyzed between pre- and post-NCPDIAC stays[16]. Only a significant increase in NK cell activity was found[16]. (D) Taken together, it was speculated that even though a short-term increase in IL-2 did not affect NK cell activity, recurrent and repeated accumulation of IL-2 for two weeks would result in a gradual enhancement of NK cell activity[16]. (E) The experimental assays were performed using peripheral blood mononuclear cells (PBMCs) from healthy volunteers, comparing changes in immune cells between exposure to a standard incubator and an NCPDIAC-resembling incubator (NC incubator; negatively charged particles were continuously forced into and out of the incubator, with negatively charged particles being predominant and accounting for approximately 3, 000 particles per 1 cc)[17]. As a result, it was found that CD25 expression in CD4+ T cells, IFNγ production in PBMC cultures, and NK cell activity were enhanced. The Immune Index also increased with the use of the NC incubator[17].
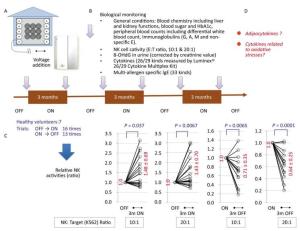
Figure Supplemental Figure 2. Long-term (three-month) stays with negatively charged particle-dominant indoor air conditions (NCPDIAC) were performed by employing the NCPDIAC device in actual living homes of seven volunteers[18]. (A) The NCPDIAC device was installed in each home and volunteers switched the device ON or OFF every three months[18]. (B) Various biomarkers were examined every three months. A total of 16 'OFF to three months ON' (ON trials) and 13 'ON to three months OFF' (OFF trials) experiments were performed with seven volunteers[18]. (C) Significant changes were only found in NK cell activity. The ON and OFF trials showed enhanced and reduced NK cell activity, respectively, with an NK cell to target cell ratio of 10:1 or 20:1. (D) The aim of this study was to examine the changes in adipokines and cytokines related to oxidative stress using samples from this long-term experiment[18].
doi: 10.3967/bes2018.044
Decrease in Serum Amyloid a Protein Levels Following Three-month Stays in Negatively Charged Particle-dominant Indoor Air Conditions
-
Abstract:
Objective The changes in serum adipokines and cytokines related to oxidative stress were examined during 3 months 'Off to On' and 'On to Off' periods using negatively charged particle-dominant indoor air conditions (NCPDIAC). Methods Seven volunteers participated in the study, which included 'OFF to 3 months ON' periods (ON trials) for a total of 16 times, and 'ON to 3 months OFF' (OFF trials) periods for a total of 13 times. Results With the exception of one case, serum amyloid A (SAA) levels decreased significantly during the ON trials. Conclusion Considering that SAA is an acute phase reactive protein such as C reactive protein (CRP), this observed decrease might indicate the prevention of cardiovascular and atherosclerotic changes, since an increase in high-sensitive CRP is associated with the subsequent detection of these events. -
Key words:
- Negatively charged particle /
- Indoor air /
- Serum amyloid A /
- Biomarker /
- Cardiovascular disease
-
Figure 1. Changes in actual measured values, relative changes in actual measured values (pre-value as 1.0), logarithmic converted values of actual values, and relative changes in values of ON trials observed for resistin (A) and IL-8 (B). For both cytokines, the relative changes in actual measured values tended to increase (0.5 < P < 1.0), although other analyses revealed no statistical significance or tendencies.
Figure 2. Changes in actual measured values, relative changes in actual measured values (pre-value as 1.0), logarithmic converted values of actual values, and relative changes in values of ON trials observed for SAA (A), and SAA with one case omitted, which showed an exceptionally marked increase (B). In all 16 ON trials, logarithmic converted actual values showed a tendency to decrease, and logarithmic converted relative changes showed a significant decrease in SAA levels (A). Additionally, with the omission of one case that showed an exceptionally marked increase in SAA, all other cases revealed a significant decrease in SAA (B).
Supplemental Figure 1. Summary of construction, short-term (2.5 h)[15] and mid-term (two weeks, nightly stays) experiments[16] with negatively charged particle-dominant indoor air conditions (NCPDIAC) and in vitro experimental approaches[17]. (A) The construction of NCPDIAC was performed by initially painting fine charcoal powder onto the walls and ceiling of a room[15]. The charcoal coating designated as Health Coat was produced by Artech Kohbou Co. Ltd. The NCPDIAC device was then completed by applying an electric voltage (72 V) between the backside of the walls and the ground[15]. This device generates a slight negative charge at the surface of the wall, which facilitates the adsorption of positively charged particles[15]. Thus, the presence of negatively charged particles predominates and is continuous (particles approximately 20 nm in diameter, and 500 particles per 1 cc of air volume)[15]. (B) The short-term (2.5 h) stay experiments comprising 60 volunteers, with each occupying a control or experimental (NCPDIAC) room, showed slight but significant increases in IL-2 after a 2.5 h stay with NCPDIAC[16]. (C) The mid-term (two weeks, nightly stays) experiments comprising 15 volunteers showed enhanced natural killer (NK) cell activity[16]. Volunteers initially occupied control rooms and thereafter moved to NCPDIAC rooms without being informed of the type of room they were occupying[16]. Differences in various biological markers were then analyzed between pre- and post-NCPDIAC stays[16]. Only a significant increase in NK cell activity was found[16]. (D) Taken together, it was speculated that even though a short-term increase in IL-2 did not affect NK cell activity, recurrent and repeated accumulation of IL-2 for two weeks would result in a gradual enhancement of NK cell activity[16]. (E) The experimental assays were performed using peripheral blood mononuclear cells (PBMCs) from healthy volunteers, comparing changes in immune cells between exposure to a standard incubator and an NCPDIAC-resembling incubator (NC incubator; negatively charged particles were continuously forced into and out of the incubator, with negatively charged particles being predominant and accounting for approximately 3, 000 particles per 1 cc)[17]. As a result, it was found that CD25 expression in CD4+ T cells, IFNγ production in PBMC cultures, and NK cell activity were enhanced. The Immune Index also increased with the use of the NC incubator[17].
Supplemental Figure 2. Long-term (three-month) stays with negatively charged particle-dominant indoor air conditions (NCPDIAC) were performed by employing the NCPDIAC device in actual living homes of seven volunteers[18]. (A) The NCPDIAC device was installed in each home and volunteers switched the device ON or OFF every three months[18]. (B) Various biomarkers were examined every three months. A total of 16 'OFF to three months ON' (ON trials) and 13 'ON to three months OFF' (OFF trials) experiments were performed with seven volunteers[18]. (C) Significant changes were only found in NK cell activity. The ON and OFF trials showed enhanced and reduced NK cell activity, respectively, with an NK cell to target cell ratio of 10:1 or 20:1. (D) The aim of this study was to examine the changes in adipokines and cytokines related to oxidative stress using samples from this long-term experiment[18].
-
[1] Redlich CA, Sparer J, Cullen MR. Sick-building syndrome. Lancet, 1997; 349, 1013-6 doi: 10.1016/S0140-6736(96)07220-0 [2] Menzies D, Bourbeau J. Building-related illnesses. N Engl J Med, 1997; 337, 1524-31. doi: 10.1056/NEJM199711203372107 [3] Hodgson M. Sick building syndrome. Occup Med, 2000; 15, 571-85. [4] Norbäck D, Edling C. Environmental, occupational, and personal factors related to the prevalence of sick building syndrome in the general population. Br J Ind Med, 1991; 48, 451-62. http://europepmc.org/abstract/MED/1854648 [5] Matsuzaka Y, Ohkubo T, Kikuti YY, et al. Association of sick building syndrome with neuropathy target esterase (NTE) activity in Japanese. Environ Toxicol, 2014; 29, 1217-26. doi: 10.1002/tox.v29.10 [6] Matsuzaka Y, Kikuti YY, Mizutani A, et al. Association study between sick building syndrome and polymorphisms of seven human detoxification genes in the Japanese. Environ Toxicol Pharmacol, 2010; 29, 190-4. doi: 10.1016/j.etap.2009.11.007 [7] Norbäck D. An update on sick building syndrome. Curr Opin Allergy Clin Immunol, 2009; 9, 55-9. doi: 10.1097/ACI.0b013e32831f8f08 [8] Burge PS. Sick building syndrome. Occup Environ Med, 2004; 61, 185-90. doi: 10.1136/oem.2003.008813 [9] Straus DC. Molds, mycotoxins, and sick building syndrome. Toxicol Ind Health, 2009; 25, 617-35. doi: 10.1177/0748233709348287 [10] Jacobs RL, Andrews CP, Coalson JJ. Hypersensitivity pneumonitis:beyond classic occupational disease-changing concepts of diagnosis and management. Ann Allergy Asthma Immunol, 2005; 95, 115-28. doi: 10.1016/S1081-1206(10)61200-8 [11] Patel AM, Ryu JH, Reed CE. Hypersensitivity pneumonitis:current concepts and future questions. J Allergy Clin Immunol, 2001; 108, 661-70. doi: 10.1067/mai.2001.119570 [12] Obayashi K, Saeki K, Kurumatani N. Independent Associations Between Nocturia and Nighttime Blood Pressure/Dipping in Elderly Individuals:The HEIJO-KYO Cohort. J Am Geriatr Soc, 2015; 63, 733-8. doi: 10.1111/jgs.2015.63.issue-4 [13] Saeki K, Obayashi K, Tone N, et al. A warmer indoor environment in the evening and shorter sleep onset latency in winter:The HEIJO-KYO study. Physiol Behav, 2015; 149, 29-34. doi: 10.1016/j.physbeh.2015.05.022 [14] Saeki K, Obayashi K, Tone N, et al. Daytime cold exposure and salt intake based on nocturnal urinary sodium excretion:A cross-sectional analysis of the HEIJO-KYO study. Physiol Behav, 2015; 152, 300-6. doi: 10.1016/j.physbeh.2015.10.015 [15] Takahashi K, Otsuki T, Mase A, et al. Negatively-charged air conditions and responses of the human psycho-neuroendocrino-immune network. Environ Int, 2008; 34, 765-72. doi: 10.1016/j.envint.2008.01.003 [16] Takahashi K, Otsuki T, Mase A, et al. Two weeks of permanence in negatively-charged air conditions causes alteration of natural killer cell function. Int J Immunopathol Pharmacol, 2009; 22, 333-42. doi: 10.1177/039463200902200210 [17] Nishimura Y, Takahashi K, Mase A, et al. Exposure to negatively charged-particle dominant air-conditions on human lymphocytes in vitro activates immunological responses. Immunobiology, 2015; 220, 1359-68. doi: 10.1016/j.imbio.2015.07.006 [18] Nishimura Y, Takahashi K, Mase A, et al. Enhancement of NK Cell Cytotoxicity Induced by Long-Term Living in Negatively Charged-Particle Dominant Indoor Air-Conditions. PLoS One, 2015; 10, e0132373. doi: 10.1371/journal.pone.0132373 [19] Wolf G. Insulin resistance and obesity:resistin, a hormone secreted by adipose tissue. Nutr Rev, 2004; 62, 389-94. doi: 10.1111/nure.2004.62.issue-10 [20] Haluzik M, Haluzikova D. The role of resistin in obesity-induced insulin resistance. Curr Opin Investig Drugs, 2006; 7, 306-11. https://www.researchgate.net/publication/7154333_The_role_of_resistin_in_obesity-induced_insulin_resistance [21] Filková M, Haluzík M, Gay S, et al. The role of resistin as a regulator of inflammation:Implications for various human pathologies. Clin Immunol, 2009; 133, 157-70. doi: 10.1016/j.clim.2009.07.013 [22] Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett, 1992; 307, 97-101. doi: 10.1016/0014-5793(92)80909-Z [23] Kunkel SL, Lukacs NW, Strieter RM. The role of interleukin-8 in the infectious process. Ann N Y Acad Sci, 1994; 730, 134-43. doi: 10.1111/nyas.1994.730.issue-1 [24] Roebuck KA. Oxidant stress regulation of IL-8 and ICAM-1 gene expression:differential activation and binding of the transcription factors AP-1 and NF-κB. Int J Mol Med, 1999; 4, 223-30. http://www.ncbi.nlm.nih.gov/pubmed/10425270 [25] Jain SK, Rains J, Croad J, et al. Curcumin supplementation lowers TNF-alpha, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-alpha, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid Redox Signal, 2009; 11, 241-9. doi: 10.1089/ars.2008.2140 [26] Ivison SM, Wang C, Himmel ME, et al. Oxidative stress enhances IL-8 and inhibits CCL20 production from intestinal epithelial cells in response to bacterial flagellin. Am J Physiol Gastrointest Liver Physiol, 2010; 299, G733-41. doi: 10.1152/ajpgi.00089.2010 [27] Paszti-Gere E, Csibrik-Nemeth E, Szeker K, et al. Acute oxidative stress affects IL-8 and TNF-α expression in IPEC-J2 porcine epithelial cells. Inflammation, 2012; 35, 994-1004. doi: 10.1007/s10753-011-9403-8 [28] Benditt EP, Hoffman JS, Eriksen N, et al. SAA, an apoprotein of HDL:its structure and function. Ann N Y Acad Sci, 1982; 389, 183-9. doi: 10.1111/nyas.1982.389.issue-1 [29] Salazar A, Pintó X, Mañá J. Serum amyloid A and high-density lipoprotein cholesterol:serum markers of inflammation in sarcoidosis and other systemic disorders. Eur J Clin Invest, 2001; 31, 1070-7. doi: 10.1046/j.1365-2362.2001.00913.x [30] Hua S, Song C, Geczy CL, et al. A role for acute-phase serum amyloid A and high-density lipoprotein in oxidative stress, endothelial dysfunction and atherosclerosis. Redox Rep, 2009; 14, 187-96. doi: 10.1179/135100009X12525712409490 [31] Malle E, Steinmetz A, Raynes JG. Serum amyloid A (SAA):an acute phase protein and apolipoprotein. Atherosclerosis, 1993; 102, 131-46. doi: 10.1016/0021-9150(93)90155-N [32] Marhaug G, Dowton SB. Serum amyloid A:an acute phase apolipoprotein and precursor of AA amyloid. Baillieres Clin Rheumatol, 1994; 8, 553-73. doi: 10.1016/S0950-3579(05)80115-3 [33] Malle E, De Beer FC. Human serum amyloid A (SAA) protein:a prominent acute-phase reactant for clinical practice. Eur J Clin Invest, 1996; 26, 427-35. doi: 10.1046/j.1365-2362.1996.159291.x [34] Willcox BJ, Abbott RD, Yano K, et al. C-reactive protein, cardiovascular disease and stroke:new roles for an old biomarker. Expert Rev Neurother, 2004; 4, 507-18. doi: 10.1586/14737175.4.3.507 [35] Di Napoli M, Schwaninger M, Cappelli R, et al. Evaluation of C-reactive protein measurement for assessing the risk and prognosis in ischemic stroke:a statement for health care professionals from the CRP Pooling Project members. Stroke, 2005; 36, 1316-29. doi: 10.1161/01.STR.0000165929.78756.ed [36] Clearfield MB. C-reactive protein:a new risk assessment tool for cardiovascular disease. J Am Osteopath Assoc, 2005; 105, 409-16. http://www.citeulike.org/group/982/article/697790 [37] Yeh ET. High-sensitivity C-reactive protein as a risk assessment tool for cardiovascular disease. Clin Cardiol, 2005; 28, 408-12. doi: 10.1002/clc.v28:9 -




 下载:
下载:



 Quick Links
Quick Links